Mobility of Lewis acids within the secondary coordination sphere: toward a model for cooperative substrate binding
- PMID: 33016291
- PMCID: PMC7606458
- DOI: 10.1039/d0cc05121g
Mobility of Lewis acids within the secondary coordination sphere: toward a model for cooperative substrate binding
Abstract
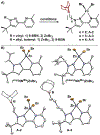
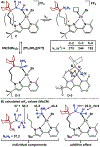
Distance dependence of appended Lewis acids in N2H4 binding and deprotonation was evaluated within a series of zinc complexes. Variation of spacer-length to a tethered trialkylborane Lewis acid revealed distinct preferences for binding and stabilization of the resulting deprotonated N2H3- unit.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures

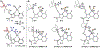
References
-
- Sawaya MR; Cannon GC; Heinhorst S; Tanaka S; Williams EB; Yeates TO; Kerfeld CA, J. Biol. Chem 2006, 281, 7546–7555; - PubMed
- Ferraroni M; Del Prete S; Vullo D; Capasso C; Supuran CT, Acta Cryst. D 2015, D71, 2449–2456; - PubMed
- Aggarwal M; Chua TK; Pinard MA; Szebenyi DM; McKenna R, Biochemistry 2015, 54, 6631–6638; - PMC - PubMed
- Cronk JD; Endrizzi JA; Cronk MR; O’Neill JW; Zhang KYJ, Protein Sci. 2001, 10, 911–922. - PMC - PubMed
-
- Miller AJM; Labinger JA; Bercaw JE, Organometallics 2010, 29, 4499–4516;
- Meng G; Lam NYS; Lucas EL; Saint-Denis TG; Verma P; Chekshin N; Yu J-Q, J. Am. Chem. Soc 2020, 142, 10571–10591; - PMC - PubMed
- Talukdar K; Sinha Roy S; Amatya E; Sleeper EA; Le Magueres P; Jurss JW, Inorg. Chem 2020, 59, 6087–6099; - PubMed
- González-Fernández R; Crochet P; Cadierno V, Dalton Trans. 2020, 49, 210–222; - PubMed
- Nichols Eva M.; Derrick JS; Nistanaki SK; Smith PT; Chang CJ, Chem. Sci 2018, 9, 2952–2960. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

