A Zwitterionic Phosphonium Stannate(II) via Hydrogen Splitting by a Sn/P Frustrated Lewis-Pair and Reductive Elimination
- PMID: 33016507
- PMCID: PMC7839681
- DOI: 10.1002/chem.202004425
A Zwitterionic Phosphonium Stannate(II) via Hydrogen Splitting by a Sn/P Frustrated Lewis-Pair and Reductive Elimination
Abstract
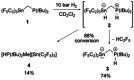
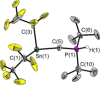
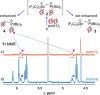
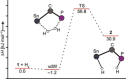
The reactivity of the frustrated Lewis pair (FLP) (F5 C2 )3 SnCH2 P(tBu)2 (1) was investigated with respect to the activation of elemental hydrogen. The reaction of 1 at elevated hydrogen pressure afforded the intramolecular phosphonium stannate(II) (F5 C2 )2 SnCH2 PH(tBu)2 (3). It was characterized by means of multinuclear NMR spectroscopy and single crystal X-ray diffraction. NMR experiments with the two isotopologues H2 and D2 showed it to be formed via an H2 adduct (F5 C2 )3 HSnCH2 PH(tBu)2 (2) and the subsequent formal reductive elimination of pentafluoroethane; this is supported by DFT calculations. Parahydrogen-induced polarization experiments revealed the formation of a second product of the reaction of 1 with H2 , [HP(tBu)2 Me][Sn(C2 F5 )3 ] (4), in 1 H NMR spectra, whereas 2 was not detected due to its transient nature.
Keywords: activation; fluoroalkyl groups; frustrated Lewis pair; hydrogen; tin.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

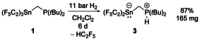

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

