β2-AR activation promotes cleavage and nuclear translocation of Her2 and metastatic potential of cancer cells
- PMID: 33016509
- PMCID: PMC7734010
- DOI: 10.1111/cas.14676
β2-AR activation promotes cleavage and nuclear translocation of Her2 and metastatic potential of cancer cells
Abstract
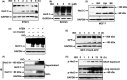
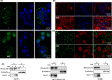
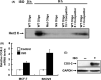
Prolonged hypersecretion of catecholamine induced by chronic stress may correlate with malignant progression of cancer. β2-adrenergic receptor (β2-AR) overexpressed in certain cancer cells may translate the signals from neuroendocrine system to malignant signals by interacting with oncoproteins, such as Her2. In the present study, we demonstrate that catecholamine stimulation activates the expression and proteolytic activity of ADAM10 by modulating the expression of miR-199a-5p and SIRT1 and also confirm that catecholamine induction triggers the activities of γ-secretase, leading to shedding of Her2 extracellular domain (ECD) by ADAM10 and subsequent intramembranous cleavage of Her2 intracellular domain (ICD) by presenilin-dependent γ-secretase, nuclear translocation of Her2 ICD, and enhanced transcription of tumor metastasis-associated gene COX-2. Chronic stimulation of catecholamine strongly promotes the invasive activities of cancer cells in vitro and spontaneous tumor lung metastasis in mice. Furthermore, nuclear localization of Her2 was significantly correlated with overexpression of β2-AR in human breast cancer tissues, indicating that catecholamine-induced β2-AR activation plays decisive roles in tumor metastasis. Our data also reveal that an unknown mechanism by which the regulated intramembrane proteolysis (RIP) initiated by β2-AR-mediated signaling controls a novel Her2-mediated signaling transduction.
Keywords: Her2; a disintegrin and metalloprotease 10; metastasis; β2-adrenergic receptor; γ-secretase.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare that there are no potential conflicts of interest.
Figures




References
-
- Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939‐944. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

