Tranexamic Acid During Prehospital Transport in Patients at Risk for Hemorrhage After Injury: A Double-blind, Placebo-Controlled, Randomized Clinical Trial
- PMID: 33016996
- PMCID: PMC7536625
- DOI: 10.1001/jamasurg.2020.4350
Tranexamic Acid During Prehospital Transport in Patients at Risk for Hemorrhage After Injury: A Double-blind, Placebo-Controlled, Randomized Clinical Trial
Erratum in
-
Errors in Figures.JAMA Surg. 2021 Jan 1;156(1):105. doi: 10.1001/jamasurg.2020.5809. JAMA Surg. 2021. PMID: 33263732 Free PMC article. No abstract available.
Abstract
Importance: In-hospital administration of tranexamic acid after injury improves outcomes in patients at risk for hemorrhage. Data demonstrating the benefit and safety of the pragmatic use of tranexamic acid in the prehospital phase of care are lacking for these patients.
Objective: To assess the effectiveness and safety of tranexamic acid administered before hospitalization compared with placebo in injured patients at risk for hemorrhage.
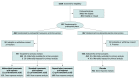
Design, setting, and participants: This pragmatic, phase 3, multicenter, double-blind, placebo-controlled, superiority randomized clinical trial included injured patients with prehospital hypotension (systolic blood pressure ≤90 mm Hg) or tachycardia (heart rate ≥110/min) before arrival at 1 of 4 US level 1 trauma centers, within an estimated 2 hours of injury, from May 1, 2015, through October 31, 2019.
Interventions: Patients received 1 g of tranexamic acid before hospitalization (447 patients) or placebo (456 patients) infused for 10 minutes in 100 mL of saline. The randomization scheme used prehospital and in-hospital phase assignments, and patients administered tranexamic acid were allocated to abbreviated, standard, and repeat bolus dosing regimens on trauma center arrival.
Main outcomes and measures: The primary outcome was 30-day all-cause mortality.
Results: In all, 927 patients (mean [SD] age, 42 [18] years; 686 [74.0%] male) were eligible for prehospital enrollment (460 randomized to tranexamic acid intervention; 467 to placebo intervention). After exclusions, the intention-to-treat study cohort comprised 903 patients: 447 in the tranexamic acid arm and 456 in the placebo arm. Mortality at 30 days was 8.1% in patients receiving tranexamic acid compared with 9.9% in patients receiving placebo (difference, -1.8%; 95% CI, -5.6% to 1.9%; P = .17). Results of Cox proportional hazards regression analysis, accounting for site, verified that randomization to tranexamic acid was not associated with a significant reduction in 30-day mortality (hazard ratio, 0.81; 95% CI, 0.59-1.11, P = .18). Prespecified dosing regimens and post-hoc subgroup analyses found that prehospital tranexamic acid were associated with significantly lower 30-day mortality. When comparing tranexamic acid effect stratified by time to treatment and qualifying shock severity in a post hoc comparison, 30-day mortality was lower when tranexamic acid was administered within 1 hour of injury (4.6% vs 7.6%; difference, -3.0%; 95% CI, -5.7% to -0.3%; P < .002). Patients with severe shock (systolic blood pressure ≤70 mm Hg) who received tranexamic acid demonstrated lower 30-day mortality compared with placebo (18.5% vs 35.5%; difference, -17%; 95% CI, -25.8% to -8.1%; P < .003).
Conclusions and relevance: In injured patients at risk for hemorrhage, tranexamic acid administered before hospitalization did not result in significantly lower 30-day mortality. The prehospital administration of tranexamic acid after injury did not result in a higher incidence of thrombotic complications or adverse events. Tranexamic acid given to injured patients at risk for hemorrhage in the prehospital setting is safe and associated with survival benefit in specific subgroups of patients.
Trial registration: ClinicalTrials.gov Identifier: NCT02086500.
Conflict of interest statement
Figures


Comment in
-
Prehospital Tranexamic Acid Administration in Injured Patients-Reply.JAMA Surg. 2021 Jul 1;156(7):688-689. doi: 10.1001/jamasurg.2021.0262. JAMA Surg. 2021. PMID: 33787837 No abstract available.
-
Prehospital Tranexamic Acid Administration in Injured Patients.JAMA Surg. 2021 Jul 1;156(7):688. doi: 10.1001/jamasurg.2021.0251. JAMA Surg. 2021. PMID: 33787843 No abstract available.
References
-
- Holcomb JB, Tilley BC, Baraniuk S, et al. ; PROPPR Study Group . Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi: 10.1001/jama.2015.12 - DOI - PMC - PubMed
-
- Disease GBD, Injury I, Prevalence C; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

