A brief history of how microscopic studies led to the elucidation of the 3D architecture and macromolecular organization of higher plant thylakoids
- PMID: 33017036
- PMCID: PMC7541383
- DOI: 10.1007/s11120-020-00782-3
A brief history of how microscopic studies led to the elucidation of the 3D architecture and macromolecular organization of higher plant thylakoids
Abstract
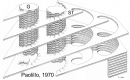
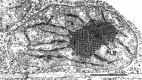
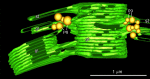
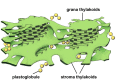
Microscopic studies of chloroplasts can be traced back to the year 1678 when Antonie van Leeuwenhoek reported to the Royal Society in London that he saw green globules in grass leaf cells with his single-lens microscope. Since then, microscopic studies have continued to contribute critical insights into the complex architecture of chloroplast membranes and how their structure relates to function. This review is organized into three chronological sections: During the classic light microscope period (1678-1940), the development of improved microscopes led to the identification of green grana, a colorless stroma, and a membrane envelope. More recent (1990-2020) chloroplast dynamic studies have benefited from laser confocal and 3D-structured illumination microscopy. The development of the transmission electron microscope (1940-2000) and thin sectioning techniques demonstrated that grana consist of stacks of closely appressed grana thylakoids interconnected by non-appressed stroma thylakoids. When the stroma thylakoids were shown to spiral around the grana stacks as multiple right-handed helices, it was confirmed that the membranes of a chloroplast are all interconnected. Freeze-fracture and freeze-etch methods verified the helical nature of the stroma thylakoids, while also providing precise information on how the electron transport chain and ATP synthase complexes are non-randomly distributed between grana and stroma membrane regions. The last section (2000-2020) focuses on the most recent discoveries made possible by atomic force microscopy of hydrated membranes, and electron tomography and cryo-electron tomography of cryofixed thylakoids. These investigations have provided novel insights into thylakoid architecture and plastoglobules (summarized in a new thylakoid model), while also producing molecular-scale views of grana and stroma thylakoids in which individual functional complexes can be identified.
Keywords: Atomic force microscopy; Chloroplasts; Electron microscopy; Electron tomography; Freeze-fracture electron microscopy; Thylakoid model.
Conflict of interest statement
No conflicts of interest and no outside funding.
Figures















References
-
- Adam Z, Charuvi D, Tsabari O, Knopf RR, Reich Z. Biogenesis of thylakoid networks in angiosperms: knowns and unknowns. Plant Mol Biol. 2011;76:221–234. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

