Quantification of Aerosol Concentrations During Endonasal Instrumentation in the Clinic Setting
- PMID: 33017067
- PMCID: PMC7675733
- DOI: 10.1002/lary.29122
Quantification of Aerosol Concentrations During Endonasal Instrumentation in the Clinic Setting
Abstract
Objective: Recent anecdotal reports and cadaveric simulations have described aerosol generation during endonasal instrumentation, highlighting a possible risk for transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) during endoscopic endonasal instrumentation. This study aims to provide a greater understanding of particle generation and exposure risk during endoscopic endonasal instrumentation.
Study design: Prospective quantification of aerosol generation during office-based nasal endoscopy procedures.
Methods: Using an optical particle sizer, airborne particles concentrations 0.3 to 10 microns in diameter, were measured during 30 nasal endoscopies in the clinic setting. Measurements were taken at time points throughout diagnostic and debridement endoscopies and compared to preprocedure and empty room particle concentrations.
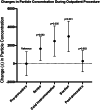
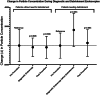
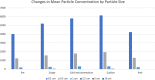
Results: No significant change in airborne particle concentrations was measured during diagnostic nasal endoscopies in patients without the need for debridement. However, significant increases in mean particle concentration compared to preprocedure levels were measured during cold instrumentation at 2,462 particles/foot3 (95% CI 837 to 4,088; P = .005) and during suction use at 2,973 particle/foot3 (95% CI 1,419 to 4,529; P = .001). In total, 99.2% of all measured particles were ≤1 μm in diameter.
Conclusion: When measured with an optical particle sizer, diagnostic nasal endoscopy with a rigid endoscope is not associated with increased particle aerosolization in patient for whom sinonasal debridement is not needed. In patients needing sinonasal debridement, endonasal cold and suction instrumentation were associated with increased particle aerosolization, with a trend observed during endoscope use prior to tissue manipulation. Endonasal debridement may potentially pose a higher risk for aerosolization and SARS-CoV-2 transmission. Appropriate personal protective equipment use and patient screening are recommended for all office-based endonasal procedures.
Level of evidence: 3 Laryngoscope, 131:E1415-E1421, 2021.
Keywords: COVID-19; aerosol-generating procedures; droplet quantification; nasal endoscopy; optical particle sizer.
© 2020 American Laryngological, Rhinological and Otological Society Inc, "The Triological Society" and American Laryngological Association (ALA).
Figures
References
-
- Nacoti M, Ciocca A, Giupponi A. At the epicenter of the Covid‐19 pandemic and humanitarian crises in Italy: changing perspectives on preparation and mitigation. NEJM Catal. 2020;1. 10.1056/CAT.20.0080. - DOI
-
- Halpern NA, Tan KS, Biostatistician AA. United States Resource Availability for COVID‐19 .
-
- Patel ZM, Fernandez‐Miranda J, Hwang PH, et al. Letter: precautions for endoscopic transnasal skull base surgery during the COVID‐19 pandemic. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7184431/. Accessed May 28, 2020. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

