Remdesivir for Adults With COVID-19 : A Living Systematic Review for American College of Physicians Practice Points
- PMID: 33017170
- PMCID: PMC7564604
- DOI: 10.7326/M20-5752
Remdesivir for Adults With COVID-19 : A Living Systematic Review for American College of Physicians Practice Points
Update in
-
Major Update: Remdesivir for Adults With COVID-19 : A Living Systematic Review and Meta-analysis for the American College of Physicians Practice Points.Ann Intern Med. 2021 May;174(5):663-672. doi: 10.7326/M20-8148. Epub 2021 Feb 9. Ann Intern Med. 2021. Update in: Ann Intern Med. 2021 Dec;174(12):W114-W115. doi: 10.7326/L21-0600. Update in: Ann Intern Med. 2022 May;175(5):701-709. doi: 10.7326/M21-4784. PMID: 33560863 Free PMC article. Updated.
Abstract
Background: Few treatments exist for coronavirus disease 2019 (COVID-19).
Purpose: To evaluate the effectiveness and harms of remdesivir for COVID-19.
Data sources: Several databases, tables of contents of journals, and U.S. Food and Drug Administration and company websites were searched from 1 January through 31 August 2020.
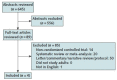
Study selection: English-language, randomized trials of remdesivir treatments for adults with suspected or confirmed COVID-19. New evidence will be incorporated using living review methods.
Data extraction: Single-reviewer abstraction and risk-of-bias assessment verified by a second reviewer; GRADE (Grading of Recommendations Assessment, Development and Evaluation) methods used for certainty-of-evidence assessments.
Data synthesis: Four randomized trials were included. In adults with severe COVID-19, remdesivir compared with placebo probably improves recovery by a large amount (absolute risk difference [ARD] range, 7% to 10%) and may result in a small reduction in mortality (ARD range, -4% to 1%) and a shorter time to recovery or clinical improvement. Remdesivir may have little to no effect on hospital length of stay. Remdesivir probably reduces serious adverse events by a moderate amount (ARD range, -6% to -8%). Compared with a 10-day remdesivir course, a 5-day course may reduce mortality, increase recovery or clinical improvement by small to moderate amounts, reduce time to recovery, and reduce serious adverse events among hospitalized patients not requiring mechanical ventilation. Recovery due to remdesivir may not vary by age, sex, symptom duration, or disease severity.
Limitations: Low-certainty evidence with few published trials, including 1 preliminary report and 2 open-label trials. Trials excluded pregnant women and adults with severe kidney or liver disease.
Conclusion: In hospitalized adults with COVID-19, remdesivir probably improves recovery and reduces serious adverse events and may reduce mortality and time to clinical improvement. For adults not receiving mechanical ventilation or extracorporeal membrane oxygenation, a 5-day course of remdesivir may provide similar benefits to and fewer harms than a 10-day course.
Primary funding source: U.S. Department of Veterans Affairs, Veterans Health Administration Office of Research and Development, Health Services Research and Development Service, and Evidence Synthesis Program.
Conflict of interest statement
References
-
- Richardson S, Hirsch JS, Narasimhan M, et al; Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052-2059. [PMID: 32320003] doi:10.1001/jama.2020.6775 - PMC - PubMed
-
- Duan-Porter W. COVID-19: intensive care unit length of stay and ventilation days. Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP project no. 09-009. 2020. Accessed at https://mfr.osf.io/render?url=https://osf.io/peyux/?direct%26mode=render... on 15 June 2020.
-
- Rome BN, Avorn J. Drug evaluation during the covid-19 pandemic. N Engl J Med. 2020;382:2282-2284. [PMID: 32289216] doi:10.1056/NEJMp2009457 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources