A microfluidic system integrated with shape memory alloy valves for a safe direct current delivery system
- PMID: 33018768
- PMCID: PMC12302988
- DOI: 10.1109/EMBC44109.2020.9176474
A microfluidic system integrated with shape memory alloy valves for a safe direct current delivery system
Abstract
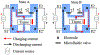
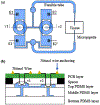
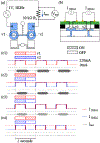
Direct current (DC) has potential as a clinical and scientific tool to accelerate wound healing, increase the permeability of the skin to drug treatment and modulate neural activity. But long duration delivery of DC unavoidably causes hazardous electrolysis at the tissue-electrode interface. To be able to deliver long duration DC, we previously proposed a design for a safe direct current stimulator (SDCS). This device uses alternating current that does not cause chemical reactions at the metal electrodes within the device, but delivers ionic direct current output to the tissue via microfluidic valves. We previously developed and published designs of multiple SDCS components including microfluidic, electronic, data processing, and energy systems. In this paper we focus on the development of the integrated microfluidics needed by the SDCS system. We developed a fabrication method and characterized valve performance within the multi-valve microfluidic system. We used poly-dimethylsiloxane (PDMS) to fabricate three microfluidic chips that integrated valves actuated by 50-µm Nitinol (NiTi) shape memory alloy (SMA) wire. We tested system operation by driving SMA valves with a current pulse and recording the valve response with an electrical assay. The valve operation complied with the SDCS system requirements. The time for valves to open was rapid at 0.177 ± 0.04 seconds, and the time for the valves to close was 0.265 ± 0.05 seconds. Open microfluidic channel impedance for unrestricted ionic current flow was 15.90 ± 8.28 kΩ and it increased by a factor of 40 to restrict ionic current flow at 678 ± 102 kΩ for the closed valves.
Figures

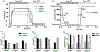
References
-
- Kloth LC and Feedar JA, “Acceleration of wound healing with high voltage, monophasic, pulsed current,” Phys. Ther, 1988. - PubMed
-
- Banga AK, Donnelly R, and Stinchcomb AL, “Transdermal drug delivery,” Therapeutic Delivery. 2013.
-
- Prausnitz MR, “The effects of electric current applied to skin: A review for transdermal drug delivery,” Advanced Drug Delivery Reviews. 1996.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
