Thalidomide in the treatment of human immunodeficiency virus-negative tuberculous meningitis: A case report
- PMID: 33019487
- PMCID: PMC7535634
- DOI: 10.1097/MD.0000000000022639
Thalidomide in the treatment of human immunodeficiency virus-negative tuberculous meningitis: A case report
Abstract
Introduction: Tuberculous meningitis (TBM) is the most fatal type of tuberculosis in which corticosteroids are added with antitubercular therapy to prevent permanent brain damage. However, this treatment may produce paradoxical reactions. In such cases, thalidomide use might reduce central nervous system inflammation and improve the outcome. We present the case of a human immunodeficiency virus-negative patient with TBM who developed paradoxical reactions manifesting as multiple intracranial tuberculomas that were resistant to standard care (antitubercular drugs and corticosteroids) but responded well to thalidomide.
Patient's main concern and clinical findings: The patient was a 40-year-old Chinese female, who was admitted with a 10-day history of headaches, night sweats, and cough. She was healthy before contracting the infection and had no history of contact with tuberculosis patients.
Diagnoses, intervention, and outcome: We diagnosed the patient with TBM complicated by the occurrence of pulmonary tuberculosis. Positive results were obtained from Gram and Ziehl-Neelsen staining of the sputum and acid-fast bacilli sputum culture. Standard treatment was initiated with antitubercular drugs (daily isoniazid, rifampicin, ethionamide, and pyrazinamide) and corticosteroids (dexamethasone). However, 3 months later the magnetic resonance imaging of the head revealed some new tuberculoma lesion. Thus, a specific therapy of antitubercular drugs and thalidomide was introduced. On completion of a 12-month course of antitubercular drugs with 2 months of thalidomide, the patient showed favorable outcomes without neurologic sequelae. Moreover, thalidomide appeared safe and well tolerated in the patient.
Conclusion: In addition to the specific anti-tubercular and adjuvant corticosteroid therapies for TBM, thalidomide can be used as a "salvage" antitubercular drug in cases that are unresponsive to corticosteroids.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

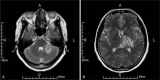
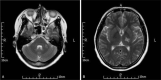
References
-
- World Health Organization. Global Tuberculosis Report 2017[R].Geneva, WHO, 2017. Available at: http://www.who.int/mediacentre/news/releases/2017/political-commitment-t....
-
- Viel-Thériault I, Thibeault R, Boucher FD, et al. Thalidomide in refractory tuberculomas and pseudoabscesses. Pediatr Infect Dis J 2016;35:1262–4. - PubMed
-
- van Toorn R, du Plessis AM, Schaaf HS, et al. Clinicoradiologic response of neurologic tuberculous mass lesions in children treated with thalidomide. Pediatr Infect Dis J 2015;34:214–8. - PubMed
-
- Thampi N, Stephens D, Rea E, et al. Unexplained deterioration during antituberculous therapy in children and adolescents: clinical presentation and risk factors. Pediatr Infect Dis J 2012;31:129–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

