Intranasal Immunization of Mice with Multiepitope Chimeric Vaccine Candidate Based on Conserved Autotransporters SigA, Pic and Sap, Confers Protection against Shigella flexneri
- PMID: 33019492
- PMCID: PMC7712744
- DOI: 10.3390/vaccines8040563
Intranasal Immunization of Mice with Multiepitope Chimeric Vaccine Candidate Based on Conserved Autotransporters SigA, Pic and Sap, Confers Protection against Shigella flexneri
Abstract
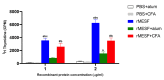
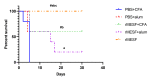
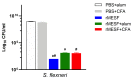
Shigellosis is a diarrheal disease and the World Health Organization prompts the development of a vaccine against Shigella flexneri. The autotransporters SigA, Pic and Sap are conserved among Shigella spp. We previously designed an in silico vaccine with immunodominat epitopes from those autotransporters, and the GroEL protein of S. typhi as an adjuvant. Here, we evaluated the immunogenicity and protective efficacy of the chimeric multiepitope protein, named rMESF, in mice against lethal infection with S. flexneri. rMESF was administered to mice alone through the intranasal (i.n.) route or accompanied with Complete Freund's adjuvant (CFA) intradermically (i.d.), subcutaneously (s.c.), and intramuscular (i.m.), as well as with Imject alum (i.m.). All immunized mice increased IgG, IgG1, IgG2a, IgA and fecal IgA titers compared to PBS+CFA and PBS+alum control groups. Furthermore, i.n. immunization of mice with rMESF alone presented the highest titers of serum and fecal IgA. Cytokine levels (IFN-γ, TNF-α, IL-4, and IL-17) and lymphocyte proliferation increased in all experimental groups, with the highest lymphoproliferative response in i.n. mice immunized with rMESF alone, which presented 100% protection against S. flexneri. In summary, this vaccine vests protective immunity and highlights the importance of mucosal immunity activation for the elimination of S. flexneri.
Keywords: GroEL; Shigella flexneri; autotransporters; bacillary dysentery; mucosal immunity; multiepitope chimeric vaccine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Kotloff K.L., Winickoff J.P., Ivanoff B., Clemens J.D., Swerdlow D.L., Sansonetti P.J., Adak G.K., Levine M.M. Global burden of Shigella infections: Implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651–666. doi: 10.1016/S0140-6736(17)33296-8. - DOI - PMC - PubMed
-
- Kozyreva V.K., Jospin G., Greninger A.L., Watt J.P., Eisen J.A., Chaturvedi V. Recent Outbreaks of Shigellosis in California Caused by Two Distinct Populations of Shigella sonnei with either Increased Virulence or Fluoroquinolone Resistance. mSphere. 2016;1:e00344-16. doi: 10.1128/mSphere.00344-16. - DOI - PMC - PubMed
-
- Oany A.R., Pervin T., Mia M., Hossain M., Shahnaij M., Mahmud S., Kibria K.M.K. Vaccinomics Approach for Designing Potential Peptide Vaccine by Targeting Shigella spp. Serine Protease Autotransporter Subfamily Protein SigA. J. Immunol Res. 2017;2017:6412353. doi: 10.1155/2017/6412353. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

