Integrins Control Vesicular Trafficking; New Tricks for Old Dogs
- PMID: 33020011
- PMCID: PMC7531435
- DOI: 10.1016/j.tibs.2020.09.001
Integrins Control Vesicular Trafficking; New Tricks for Old Dogs
Abstract
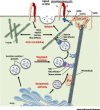
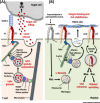
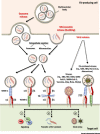
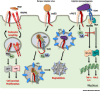
Integrins are transmembrane receptors that transduce biochemical and mechanical signals across the plasma membrane and promote cell adhesion and migration. In addition, integrin adhesion complexes are functionally and structurally linked to components of the intracellular trafficking machinery and accumulating data now reveal that they are key regulators of endocytosis and exocytosis in a variety of cell types. Here, we highlight recent insights into integrin control of intracellular trafficking in processes such as degranulation, mechanotransduction, cell-cell communication, antibody production, virus entry, Toll-like receptor signaling, autophagy, and phagocytosis, as well as the release and uptake of extracellular vesicles. We discuss the underlying molecular mechanisms and the implications for a range of pathophysiological contexts, including hemostasis, immunity, tissue repair, cancer, and viral infection.
Keywords: clathrin; endocytosis; exocytosis; immunity; integrins; viral infection.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

