How achievable are COVID-19 clinical trial recruitment targets? A UK observational cohort study and trials registry analysis
- PMID: 33020111
- PMCID: PMC7536634
- DOI: 10.1136/bmjopen-2020-044566
How achievable are COVID-19 clinical trial recruitment targets? A UK observational cohort study and trials registry analysis
Abstract
Objectives: To analyse enrolment to interventional trials during the first wave of the COVID-19 pandemic in England and describe the barriers to successful recruitment in the circumstance of a further wave or future pandemics.
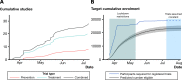
Design: We analysed registered interventional COVID-19 trial data and concurrently did a prospective observational study of hospitalised patients with COVID-19 who were being assessed for eligibility to one of the RECOVERY, C19-ACS or SIMPLE trials.
Setting: Interventional COVID-19 trial data were analysed from the clinicaltrials.gov and International Standard Randomized Controlled Trial Number databases on 12 July 2020. The patient cohort was taken from five centres in a respiratory National Institute for Health Research network. Population and modelling data were taken from published reports from the UK government and Medical Research Council Biostatistics Unit.
Participants: 2082 consecutive admitted patients with laboratory-confirmed SARS-CoV-2 infection from 27 March 2020 were included.
Main outcome measures: Proportions enrolled, and reasons for exclusion from the aforementioned trials. Comparisons of trial recruitment targets with estimated feasible recruitment numbers.
Results: Analysis of trial registration data for COVID-19 treatment studies enrolling in England showed that by 12 July 2020, 29 142 participants were needed. In the observational study, 430 (20.7%) proceeded to randomisation. 82 (3.9%) declined participation, 699 (33.6%) were excluded on clinical grounds, 363 (17.4%) were medically fit for discharge and 153 (7.3%) were receiving palliative care. With 111 037 people hospitalised with COVID-19 in England by 12 July 2020, we determine that 22 985 people were potentially suitable for trial enrolment. We estimate a UK hospitalisation rate of 2.38%, and that another 1.25 million infections would be required to meet recruitment targets of ongoing trials.
Conclusions: Feasible recruitment rates, study design and proliferation of trials can limit the number, and size, that will successfully complete recruitment. We consider that fewer, more appropriately designed trials, prioritising cooperation between centres would maximise productivity in a further wave.
Keywords: COVID-19; clinical trials; infectious diseases.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Norwegian Coronavirus Disease 2019 (NO COVID-19) Pragmatic Open label Study to assess early use of hydroxychloroquine sulphate in moderately severe hospitalised patients with coronavirus disease 2019: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 5;21(1):485. doi: 10.1186/s13063-020-04420-0. Trials. 2020. PMID: 32503662 Free PMC article.
-
Subcutaneous Sarilumab in hospitalised patients with moderate-severe COVID-19 infection compared to the standard of care (SARCOVID): a structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Sep 9;21(1):772. doi: 10.1186/s13063-020-04588-5. Trials. 2020. PMID: 32907638 Free PMC article.
-
A Randomized Open label Phase-II Clinical Trial with or without Infusion of Plasma from Subjects after Convalescence of SARS-CoV-2 Infection in High-Risk Patients with Confirmed Severe SARS-CoV-2 Disease (RECOVER): A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 6;21(1):828. doi: 10.1186/s13063-020-04735-y. Trials. 2020. PMID: 33023671 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Jul 10;7(7):CD013600. doi: 10.1002/14651858.CD013600.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. PMID: 32648959 Free PMC article. Updated.
Cited by
-
Elicitation of quantitative, choice-based preferences for Person-Centered Care among People living with Dementia in comparison to physicians' judgements in Germany: study protocol for the mixed-methods PreDemCare-study.BMC Geriatr. 2022 Jul 8;22(1):567. doi: 10.1186/s12877-022-03238-6. BMC Geriatr. 2022. PMID: 35804302 Free PMC article.
-
How informative were early SARS-CoV-2 treatment and prevention trials? a longitudinal cohort analysis of trials registered on ClinicalTrials.gov.PLoS One. 2022 Jan 21;17(1):e0262114. doi: 10.1371/journal.pone.0262114. eCollection 2022. PLoS One. 2022. PMID: 35061758 Free PMC article.
-
Clinical trial research on COVID-19 in Germany - a systematic analysis.F1000Res. 2021 Sep 10;10:913. doi: 10.12688/f1000research.55541.1. eCollection 2021. F1000Res. 2021. PMID: 38144171 Free PMC article.
-
General practice attendances among patients attending a post-COVID-19 clinic: a pilot study.BJGP Open. 2021 Jun 30;5(3):BJGPO.2021.0016. doi: 10.3399/BJGPO.2021.0016. Print 2021 Jun. BJGP Open. 2021. PMID: 33757962 Free PMC article.
-
Clinical Trial Data Sharing for COVID-19-Related Research.J Med Internet Res. 2021 Mar 12;23(3):e26718. doi: 10.2196/26718. J Med Internet Res. 2021. PMID: 33684053 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
