Efficacy of Three Antiretroviral Regimens Initiated during Pregnancy: Clinical Experience in Rio de Janeiro
- PMID: 33020151
- PMCID: PMC7674033
- DOI: 10.1128/AAC.01068-20
Efficacy of Three Antiretroviral Regimens Initiated during Pregnancy: Clinical Experience in Rio de Janeiro
Abstract
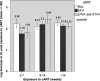
Few studies have compared the clinical efficacy and adverse events of combined antiretroviral therapy (cART) regimens in pregnant women seeking obstetrical care. The objective of this study was to compare the efficacy (virus load response), adverse events, and obstetrical and neonatal outcomes of three different regimens of cART in HIV-infected pregnant women initiating treatment in Rio de Janeiro, Brazil. This was a retrospective cohort study of cART-naive pregnant women who initiated either ritonavir-boosted protease inhibitors (atazanavir or lopinavir), efavirenz, or raltegravir plus a backbone regimen. From 2014 to 2018, 390 pregnant women were followed over time. At baseline, the median viral load (VL) for HIV was 4.1 log copies/ml. Among participants who received cART for 2 to 7 weeks, the VL decline was greater for raltegravir (2.24 log copies/ml) than for efavirenz or protease inhibitors (P < 0.001). Virologic suppression was achieved in 87% of women on raltegravir near delivery versus 73% on efavirenz and 70% on protease inhibitors (P = 0.011). Patients on raltegravir achieved virologic suppression faster than those on other regimens (P = 0.019). Overall, the HIV perinatal infection rate was 1.5%. This clinical study compared three potent and well-tolerated cART regimens and demonstrated that a higher proportion of participants on raltegravir achieved an undetectable HIV VL near delivery (P = 0.011) compared to the other arms. These findings suggest that raltegravir-containing regimens are optimal regimens for women with HIV initiating treatment late in pregnancy.
Keywords: HIV; cesarean; efavirenz; integrase inhibitors; mother-to-child transmission; nonnucleotide reverse transcriptase inhibitors; obstetrics; perinatal transmission; pregnancy; protease inhibitors; raltegravir.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- U.S. Department of Health and Human Services. 2020. The global HIV/AIDS epidemic—data and trends: global statistics. U.S. Department of Health and Human Services, Washington, DC: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics. Accessed 22 September 2020.
-
- Bartlett J, Redfield R, Pham P. 2019. Bartlett’s medical management of HIV infection. Oxford University Press, Oxford, United Kingdom.
-
- British HIV Association. 2019. Management of HIV Infection in pregnancy and postpartum 2018 (2019 interim update). BHIVA, London, United Kingdom.
-
- WHO. 2018. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection World Health Organization, Geneva, Switzerland.
-
- AIDSInfo. 2019. Panel on Antiretroviral Guidelines for Adults and Adolescents: guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services, Washington, DC.

