Piperaquine Exposure Is Altered by Pregnancy, HIV, and Nutritional Status in Ugandan Women
- PMID: 33020153
- PMCID: PMC7674024
- DOI: 10.1128/AAC.01013-20
Piperaquine Exposure Is Altered by Pregnancy, HIV, and Nutritional Status in Ugandan Women
Abstract
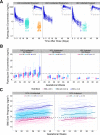
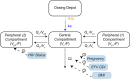
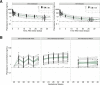
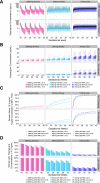
Dihydroartemisinin-piperaquine (DHA-PQ) provides highly effective therapy and chemoprevention for malaria in pregnant African women. PQ concentrations of >10.3 ng/ml have been associated with reduced maternal parasitemia, placental malaria, and improved birth outcomes. We characterized the population pharmacokinetics (PK) of PQ in a post hoc analysis of human immunodeficiency virus (HIV)-infected and -uninfected pregnant women receiving DHA-PQ as chemoprevention every 4 or 8 weeks. The effects of covariates such as pregnancy, nutritional status (body mass index [BMI]), and efavirenz (EFV)-based antiretroviral therapy were investigated. PQ concentrations from two chemoprevention trials were pooled to create a population PK database from 274 women and 2,218 PK observations. A three-compartment model with an absorption lag best fit the data. Consistent with our prior intensive PK evaluation, pregnancy and EFV use resulted in a 72% and 61% increased PQ clearance, compared to postpartum and HIV-uninfected pregnant women, respectively. Low BMI at 28 weeks of gestation was associated with increased clearance (2% increase per unit decrease in BMI). Low-BMI women given DHA-PQ every 8 weeks had a higher prevalence of parasitemia, malaria infection, and placental malaria compared to women with higher BMIs. The reduced piperaquine exposure in women with low BMI as well as during EFV coadministration, compared to pregnant women with higher BMIs and not taking EFV, suggests that these populations could benefit from weekly instead of monthly dosing for prevention of malaria parasitemia. Simulations indicated that because of the BMI-clearance relationship, weight-based regimens would not improve protection compared to a 2,880 mg fixed-dose regimen when provided monthly. (The clinical trials described in this paper have been registered at ClinicalTrials.gov under identifiers NCT02163447 and NCT02282293.).
Keywords: dihydroartemisinin-piperaquine; drug-drug interactions; malaria prevention; pharmacokinetics; population pharmacokinetics; pregnancy.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- World Health Organization. 2019. World Malaria Report 2019. World Health Organization, Geneva, Switzerland: https://www.who.int/publications/i/item/9789241565721.
-
- Akinyemi RO, Izzeldin IM, Dotchin C, Gray WK, Adeniji O, Seidi OA, Mwakisambwe JJ, Mhina CJ, Mutesi F, Msechu HZ, Mteta KA, Ahmed MA, Hamid SH, Abuelgasim NA, Mohamed SA, Mohamed AY, Adesina F, Hamzat M, Olunuga T, Maro VP, Walker R. 2014. Contribution of noncommunicable diseases to medical admissions of elderly adults in Africa: a prospective, cross-sectional study in Nigeria, Sudan, and Tanzania. J Am Geriatr Soc 62:1460–1466. doi: 10.1111/jgs.12940. - DOI - PubMed
-
- Moore KA, Fowkes FJI, Wiladphaingern J, Wai NS, Paw MK, Pimanpanarak M, Carrara VI, Raksuansak J, Simpson JA, White NJ, Nosten F, McGready R. 2017. Mediation of the effect of malaria in pregnancy on stillbirth and neonatal death in an area of low transmission: observational data analysis. BMC Med 15:98. doi: 10.1186/s12916-017-0863-z. - DOI - PMC - PubMed

