Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells
- PMID: 33020399
- PMCID: PMC7601246
- DOI: 10.3390/biom10101404
Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells
Abstract
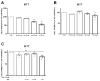
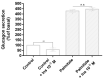
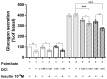
The insulin resistance state of pancreatic α-cells seems to be related to glucagon hypersecretion in type 2 diabetes. Treatment that can improve the insulin sensitivity of α-cells could control glucagon levels in patients with diabetes mellitus. The aim of this study was to investigate the preventive role of D-chiro-inositol (DCI), which has insulin receptor-sensitizer effects on insulin signaling pathways and glucagon secretion in pancreatic α-TC1 clone 6 cells. Cells were chronically treated with palmitate to induce insulin resistance in the presence/absence of DCI. DCI treatment improved the insulin signaling pathway and restored insulin-mediated glucagon suppression in α-TC1-6 cells exposed to palmitate. These results indicate that DCI treatment prevents the insulin resistance of α-TC1-6 cells chronically exposed to palmitate. Our data provide evidence that DCI could be useful to improve the insulin sensitivity of pancreatic α-cells in diabetes treatment.
Keywords: D-chiro-inositol; insulin resistance; lipotoxicity; α-cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

