HDAC6 Regulates Radiosensitivity of Non-Small Cell Lung Cancer by Promoting Degradation of Chk1
- PMID: 33020410
- PMCID: PMC7600810
- DOI: 10.3390/cells9102237
HDAC6 Regulates Radiosensitivity of Non-Small Cell Lung Cancer by Promoting Degradation of Chk1
Abstract
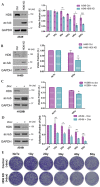
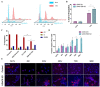
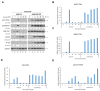
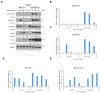
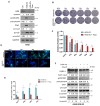
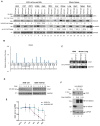
We have previously discovered that HDAC6 regulates the DNA damage response (DDR) via modulating the homeostasis of a DNA mismatch repair protein, MSH2, through HDAC6's ubiquitin E3 ligase activity. Here, we have reported HDAC6's second potential E3 ligase substrate, a critical cell cycle checkpoint protein, Chk1. We have found that HDAC6 and Chk1 directly interact, and that HDAC6 ubiquitinates Chk1 in vivo and in vitro. Specifically, HDAC6 interacts with Chk1 via the DAC1 domain, which contains its ubiquitin E3 ligase activity. During the cell cycle, Chk1 protein levels fluctuate, peaking at the G2 phase, subsequently resolving via the ubiquitin-proteasome pathway, and thereby allowing cells to progress to the M phase. However, in HDAC6 knockdown non-small cell lung cancer (NSCLC) cells, Chk1 is constitutively active and fails to resolve post-ionizing radiation (IR), and this enhanced Chk1 activity leads to preferential G2 arrest in HDAC6 knockdown cells accompanied by a reduction in colony formation capacity and viability. Depletion or pharmacological inhibition of Chk1 in HDAC6 knockdown cells reverses this radiosensitive phenotype, suggesting that the radiosensitivity of HDAC6 knockdown cells is dependent on increased Chk1 kinase activity. Overall, our results highlight a novel mechanism of Chk1 regulation at the post-translational level, and a possible strategy for sensitizing NSCLC to radiation via inhibiting HDAC6's E3 ligase activity.
Keywords: DNA damage response (DDR); checkpoint kinase 1 (Chk1); histone deacetylase 6 (HDAC6); ionizing radiation (IR); ubiquitin E3 ligase; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

