Liver Biopsy Hydroxyproline Content Is a Diagnostic for Hepatocellular Carcinoma in Murine Models of Nonalcoholic Steatohepatitis
- PMID: 33020436
- PMCID: PMC7601536
- DOI: 10.3390/diagnostics10100784
Liver Biopsy Hydroxyproline Content Is a Diagnostic for Hepatocellular Carcinoma in Murine Models of Nonalcoholic Steatohepatitis
Abstract
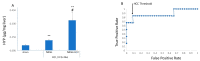
There is increasing evidence that nonalcoholic steatohepatitis (NASH) is a risk factor for hepatocellular carcinoma (HCC) in the absence of cirrhosis, a phenomenon termed noncirrhotic HCC. Early diagnosis of HCC is critical to a favorable prognosis. We tested the hypothesis that hydroxyproline content of liver biopsy samples is diagnostic for HCC in murine models of NASH induced by diet or by diet and chemicals. The training set comprised mice fed a standard diet or a fast-food diet with or without administration of thioacetamide. At harvest, livers from the modified diet cohort exhibited NASH with a subset of NASH livers exhibiting HCC. Hydroxyproline content was measured in liver biopsy samples with tissue in the NASH+HCC cohort sampled from the remote, nontumor parenchyma. Plotting the receiver operating characteristics (ROC) with hydroxyproline as the continuous variable against the absence or presence of HCC yielded an area under ROC of 0.87, a threshold of >0.18 μg hydroxyproline/mg liver and sensitivity of 91% with a specificity of 83.3%. The use of liver hydroxyproline content as a diagnostic for HCC in a test set comprising healthy, NASH and NASH+HCC livers proved 87% accurate.
Keywords: HCC; NASH; ROC; cancer; diagnostic; fatty; hydroxyproline; liver; nonalcoholic.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Sanyal A.J., Friedman S.L., McCullough A.J., Dimick-Santos L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: Findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. American Association for the Study of Liver Diseases; United States Food and Drug Administration. Hepatology. 2015;61:1392–1405. - PMC - PubMed
-
- Hwang A., Shi C., Zhu E., Naaz F., Zhou P., Rasheed Z., Liu M., Jung L.S., Duan B., Li J., et al. Supervised Learning Reveals Circulating Biomarker Levels Diagnostic of Hepatocellular Carcinoma in a Clinically Relevant Model of Non-alcoholic Steatohepatitis; An OAD to NASH. PLoS ONE. 2018;13:e0198937. doi: 10.1371/journal.pone.0198937. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

