The Role of Hypoxia and SRC Tyrosine Kinase in Glioblastoma Invasiveness and Radioresistance
- PMID: 33020459
- PMCID: PMC7599682
- DOI: 10.3390/cancers12102860
The Role of Hypoxia and SRC Tyrosine Kinase in Glioblastoma Invasiveness and Radioresistance
Abstract
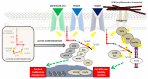
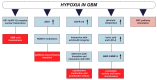
Advances in functional imaging are supporting neurosurgery and radiotherapy for glioblastoma, which still remains the most aggressive brain tumor with poor prognosis. The typical infiltration pattern of glioblastoma, which impedes a complete surgical resection, is coupled with a high rate of invasiveness and radioresistance, thus further limiting efficient therapy, leading to inevitable and fatal recurrences. Hypoxia is of crucial importance in gliomagenesis and, besides reducing radiotherapy efficacy, also induces cellular and molecular mediators that foster proliferation and invasion. In this review, we aimed at analyzing the biological mechanism of glioblastoma invasiveness and radioresistance in hypoxic niches of glioblastoma. We also discussed the link between hypoxia and radiation-induced radioresistance with activation of SRC proto-oncogene non-receptor tyrosine kinase, prospecting potential strategies to overcome the current limitation in glioblastoma treatment.
Keywords: Glioblastoma; SRC tyrosine kinase; hypoxia; invasion; radioresistance; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wen P.Y., Weller M., Lee E.Q., A Alexander B., Barnholtz-Sloan J.S., Barthel F.P., Batchelor T.T., Bindra R.S., Chang S.M., Chiocca E.A., et al. Glioblastoma in Adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) Consensus Review on Current Management and Future Directions. Neuro-Oncology. 2020;22:1073–1113. doi: 10.1093/neuonc/noaa106. - DOI - PMC - PubMed
-
- Verhaak R.G., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. - DOI - PMC - PubMed
-
- Louis D.N., Perry A., Reifenberger G., Von Deimling A., Figarella-Branger M., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Cabrera A.R., Kirkpatrick J., Fiveash J.B., Shih H.A., Koay E.J., Lutz S., Petit J., Chao S.T., Brown P.D., Vogelbaum M., et al. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Prac. Radiat. Oncol. 2016;6:217–225. doi: 10.1016/j.prro.2016.03.007. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

