Three-dimensional optoacoustic imaging of nailfold capillaries in systemic sclerosis and its potential for disease differentiation using deep learning
- PMID: 33020505
- PMCID: PMC7536218
- DOI: 10.1038/s41598-020-73319-2
Three-dimensional optoacoustic imaging of nailfold capillaries in systemic sclerosis and its potential for disease differentiation using deep learning
Abstract
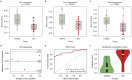
The autoimmune disease systemic sclerosis (SSc) causes microvascular changes that can be easily observed cutaneously at the finger nailfold. Optoacoustic imaging (OAI), a combination of optical and ultrasound imaging, specifically raster-scanning optoacoustic mesoscopy (RSOM), offers a non-invasive high-resolution 3D visualization of capillaries allowing for a better view of microvascular changes and an extraction of volumetric measures. In this study, nailfold capillaries of patients with SSc and healthy controls are imaged and compared with each other for the first time using OAI. The nailfolds of 23 patients with SSc and 19 controls were imaged using RSOM. The acquired images were qualitatively compared to images from state-of-the-art imaging tools for SSc, dermoscopy and high magnification capillaroscopy. The vascular volume in the nailfold capillaries were computed from the RSOM images. The vascular volumes differ significantly between both cohorts (0.216 ± 0.085 mm3 and 0.337 ± 0.110 mm3; p < 0.0005). In addition, an artificial neural network was trained to automatically differentiate nailfold images from both cohorts to further assess whether OAI is sensitive enough to visualize anatomical differences in the capillaries between the two cohorts. Using transfer learning, the model classifies images with an area under the ROC curve of 0.897, and a sensitivity of 0.783 and specificity of 0.895. In conclusion, this study demonstrates the capabilities of RSOM as an imaging tool for SSc and establishes it as a modality that facilitates more in-depth studies into the disease mechanisms and progression.
Conflict of interest statement
K.H. is an employee of iThera Medical. V.N. is a co-founder and shareholder of iThera Medical. Authors S.N., T.B.M, J.B.M., G.D., M.B, C.T., M.R.D., D.J. A.L.H and A.K.M declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

