Differential reperfusion patterns in retinal vascular plexuses following increase in intraocular pressure an OCT angiography study
- PMID: 33020568
- PMCID: PMC7536233
- DOI: 10.1038/s41598-020-73585-0
Differential reperfusion patterns in retinal vascular plexuses following increase in intraocular pressure an OCT angiography study
Abstract
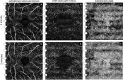
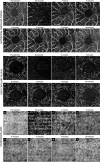
To describe patterns of reperfusion in the superficial vascular plexus (SVP), deep capillary plexus (DCP) and choriocapillaris (CC) as detected on optical coherence tomography (OCTA) in cynomogulus macaque monkey model following increase in intraocular pressure by an intravitreal injection. Animal imaging study. Two cynomogulus macaque monkeys. A 100 µL intravitreal injection (IVI) of saline was given in one eye of each monkey. Serial OCTA using a Zeiss Plex Elite 9000 was used to evaluate reperfusion patterns within the SCP, DCP, and CC. OCTA evidence of perfusion. Pulsation of the central retinal artery was detected after the intraocular pressure was elevated to 98 and ≥ 99 mmHg from IVI. Episodic flow within the SVP arterioles and venules and poor visualization of flow in capillaries was noted during the initial phase of elevated pressure. As the pressure declined, the flow signal within the DCP appeared initially as dots, which progressed laterally to loops which form capillary vortex configuration. Recovery of flow within the SVP and CC appeared sooner than in the DCP. At 40 min after the injection, well after the intraocular pressure normalized, the retinal and choriocapillaris vascular perfusion showed focal defects in every layer. Compared with pre-injection images, vessel density in the DCP was 68.8% and 78.6% of baseline in monkey 1 and monkey 2, respectively. In contrast vessel density in the SVP recovered to 84.2% and 88.9% of baseline. Increases in intraocular pressure from IVI have the potential to affect every layer of blood flow in the fundus. After nominal return of intraocular pressure, focal defects in flow persisted, which may result in longer term damage to the retina.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

