Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas
- PMID: 33020644
- PMCID: PMC8446909
- DOI: 10.1038/s41591-020-1061-7
Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas
Abstract
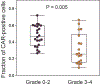
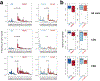
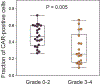
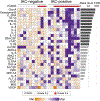
Autologous chimeric antigen receptor (CAR) T cell therapies targeting CD19 have high efficacy in large B cell lymphomas (LBCLs), but long-term remissions are observed in less than half of patients, and treatment-associated adverse events, such as immune effector cell-associated neurotoxicity syndrome (ICANS), are a clinical challenge. We performed single-cell RNA sequencing with capture-based cell identification on autologous axicabtagene ciloleucel (axi-cel) anti-CD19 CAR T cell infusion products to identify transcriptomic features associated with efficacy and toxicity in 24 patients with LBCL. Patients who achieved a complete response by positron emission tomography/computed tomography at their 3-month follow-up had three-fold higher frequencies of CD8 T cells expressing memory signatures than patients with partial response or progressive disease. Molecular response measured by cell-free DNA sequencing at day 7 after infusion was significantly associated with clinical response (P = 0.008), and a signature of CD8 T cell exhaustion was associated (q = 2.8 × 10-149) with a poor molecular response. Furthermore, a rare cell population with monocyte-like transcriptional features was associated (P = 0.0002) with high-grade ICANS. Our results suggest that heterogeneity in the cellular and molecular features of CAR T cell infusion products contributes to variation in efficacy and toxicity after axi-cel therapy in LBCL, and that day 7 molecular response might serve as an early predictor of CAR T cell efficacy.
Figures






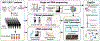


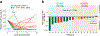


Comment in
-
CAR T-cell Characteristics Determine Efficacy.Cancer Discov. 2020 Dec;10(12):1780. doi: 10.1158/2159-8290.CD-NB2020-096. Epub 2020 Oct 23. Cancer Discov. 2020. PMID: 33097479
-
Using single-cell analysis to predict CAR T cell outcomes.Nat Med. 2020 Dec;26(12):1813-1814. doi: 10.1038/s41591-020-01157-w. Nat Med. 2020. PMID: 33230341 No abstract available.
References
Methods-only References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

