Continuous versus intermittent BRAF and MEK inhibition in patients with BRAF-mutated melanoma: a randomized phase 2 trial
- PMID: 33020646
- PMCID: PMC8063889
- DOI: 10.1038/s41591-020-1060-8
Continuous versus intermittent BRAF and MEK inhibition in patients with BRAF-mutated melanoma: a randomized phase 2 trial
Abstract
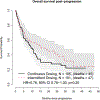
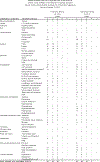
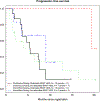
Preclinical modeling suggests that intermittent BRAF inhibitor therapy may delay acquired resistance when blocking oncogenic BRAFV600 in melanoma1,2. We conducted S1320, a randomized, open-label, phase 2 clinical trial (NCT02196181) evaluating whether intermittent dosing of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib improves progression-free survival in patients with metastatic and unresectable BRAFV600 melanoma. Patients were enrolled at 68 academic and community sites nationally. All patients received continuous dabrafenib and trametinib during an 8-week lead-in period, after which patients with non-progressing tumors were randomized to either continuous or intermittent dosing of both drugs on a 3-week-off, 5-week-on schedule. The trial has completed accrual and 206 patients with similar baseline characteristics were randomized 1:1 to the two study arms (105 to continuous dosing, 101 to intermittent dosing). Continuous dosing yielded a statistically significant improvement in post-randomization progression-free survival compared with intermittent dosing (median 9.0 months versus 5.5 months, P = 0.064, pre-specified two-sided α = 0.2). Therefore, contrary to the initial hypothesis, intermittent dosing did not improve progression-free survival in patients. There were no differences in the secondary outcomes, including overall survival and the overall incidence of treatment-associated toxicity, between the two groups.
Figures





References
-
- Ascierto PA et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 17, 1248–1260 (2016). - PubMed
-
- Long GV et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet Lond. Engl. 386, 444–451 (2015). - PubMed
-
- Dummer R. et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 19, 1315–1327 (2018). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA189829/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- U10 CA180834/CA/NCI NIH HHS/United States
- UG1 CA189821/CA/NCI NIH HHS/United States
- UG1 CA233230/CA/NCI NIH HHS/United States
- UG1 CA233330/CA/NCI NIH HHS/United States
- UG1 CA189822/CA/NCI NIH HHS/United States
- UG1 CA233324/CA/NCI NIH HHS/United States
- R35 CA197633/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- P01 CA244118/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- UG1 CA189808/CA/NCI NIH HHS/United States
- UG1 CA189957/CA/NCI NIH HHS/United States
- UG1 CA189858/CA/NCI NIH HHS/United States
- UG1 CA189860/CA/NCI NIH HHS/United States
- UG1 CA189830/CA/NCI NIH HHS/United States
- UG1 CA189954/CA/NCI NIH HHS/United States
- UG1 CA189809/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- UG1 CA239767/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA189958/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- UG1 CA189953/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

