Charge transfer reaction mechanisms of epoxyketone and boronated peptides at glassy carbon and boron doped diamond electrodes
- PMID: 33020701
- PMCID: PMC7526604
- DOI: 10.1016/j.jelechem.2020.114733
Charge transfer reaction mechanisms of epoxyketone and boronated peptides at glassy carbon and boron doped diamond electrodes
Abstract
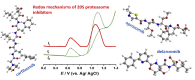
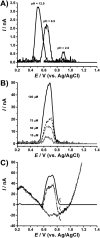
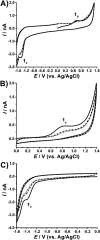
The ubiquitin-proteasome system regulates the level of proteins within cells through controlled proteolysis. In some diseases, the system function is dysregulated turning the ubiquitin-proteasome complex into a target for drug development. The redox behavior of proteasome inhibitors, epoxyketone and boronated peptides carfilzomib, oprozomib and delanzomib was investigated by voltammetric methods using glassy carbon and boron doped diamond electrodes. It was showed that the oxidation of epoxyketone peptides carfilzomib and oprozomib occurred in one step at glassy carbon electrode surface while at boron doped diamond two consecutive charge transfer reactions due to different adsorption orientation at the electrode surface were observed. The moieties of these peptides, involved in the oxidation process, were morpholine for carfilzomib and thiazole for oprozomib. For the boronated peptide delanzomib, two irreversible and independent redox processes, oxidation at +0.80 V and reduction at -1.40 V were identified in neutral media at both electrodes. The oxidation reaction occurred at the amino group close to the pyridine moiety of delanzomib with the transfer of one electron and one proton whereas the reduction process takes place at pyridine ring in a two-electrons two-protons mechanism. Redox mechanisms were proposed and the implications on the proteasome inhibition discussed.
Keywords: Boron doped diamond; Carfilzomib; Delanzomib; Glassy carbon; Oprozomib; Redox mechanism.
© 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
LinkOut - more resources
Full Text Sources
