Optical control of ERK and AKT signaling promotes axon regeneration and functional recovery of PNS and CNS in Drosophila
- PMID: 33021199
- PMCID: PMC7567606
- DOI: 10.7554/eLife.57395
Optical control of ERK and AKT signaling promotes axon regeneration and functional recovery of PNS and CNS in Drosophila
Abstract
Neuroregeneration is a dynamic process synergizing the functional outcomes of multiple signaling circuits. Channelrhodopsin-based optogenetics shows the feasibility of stimulating neural repair but does not pin down specific signaling cascades. Here, we utilized optogenetic systems, optoRaf and optoAKT, to delineate the contribution of the ERK and AKT signaling pathways to neuroregeneration in live Drosophila larvae. We showed that optoRaf or optoAKT activation not only enhanced axon regeneration in both regeneration-competent and -incompetent sensory neurons in the peripheral nervous system but also allowed temporal tuning and proper guidance of axon regrowth. Furthermore, optoRaf and optoAKT differ in their signaling kinetics during regeneration, showing a gated versus graded response, respectively. Importantly in the central nervous system, their activation promotes axon regrowth and functional recovery of the thermonociceptive behavior. We conclude that non-neuronal optogenetics targets damaged neurons and signaling subcircuits, providing a novel strategy in the intervention of neural damage with improved precision.
Keywords: D. melanogaster; Drosophila; axon regeneration; dendritic arborization; neuroscience; optogenetics; sensory neurons; thermonociception.
Plain language summary
Most cells have a built-in regeneration signaling program that allows them to divide and repair. But, in the cells of the central nervous system, which are called neurons, this program is ineffective. This is why accidents and illnesses affecting the brain and spinal cord can cause permanent damage. Reactivating regeneration in neurons could help them repair, but it is not easy. Certain small molecules can switch repair signaling programs back on. Unfortunately, these molecules diffuse easily through tissues, spreading around the body and making it hard to target individual damaged cells. This both hampers research into neuronal repair and makes treatments directed at healing damage to the nervous system more likely to have side-effects. It is unclear whether reactivating regeneration signaling in individual neurons is possible. One way to address this question is to use optogenetics. This technique uses genetic engineering to fuse proteins that are light-sensitive to proteins responsible for relaying signals in the cell. When specific wavelengths of light hit the light-sensitive proteins, the fused signaling proteins switch on, leading to the activation of any proteins they control, for example, those involved in regeneration. Wang et al. used optogenetic tools to determine if light can help repair neurons in fruit fly larvae. First, a strong laser light was used to damage an individual neuron in a fruit fly larva that had been genetically modified so that blue light would activate the regeneration program in its neurons. Then, Wang et al. illuminated the cell with dim blue light, switching on the regeneration program. Not only did this allow the neuron to repair itself, it also allowed the light to guide its regeneration. By focusing the blue light on the damaged end of the neuron, it was possible to guide the direction of the cell's growth as it regenerated. Regeneration programs in flies and mammals involve similar signaling proteins, but blue light does not penetrate well into mammalian tissues. This means that further research into LEDs that can be implanted may be necessary before neuronal repair experiments can be performed in mammals. In any case, the ability to focus treatment on individual neurons paves the way for future work into the regeneration of the nervous system, and the combination of light and genetics could reveal more about how repair signals work.
© 2020, Wang et al.
Conflict of interest statement
QW, HF, FL, SS, VK, YS, KZ No competing interests declared
Figures
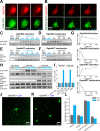
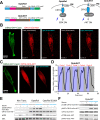





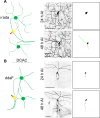
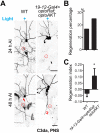




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

