Total Synthesis of (-)-Mitrephorone A Enabled by Stereoselective Nitrile Oxide Cycloaddition and Tetrasubstituted Olefin Synthesis
- PMID: 33021371
- PMCID: PMC7564100
- DOI: 10.1021/jacs.0c09520
Total Synthesis of (-)-Mitrephorone A Enabled by Stereoselective Nitrile Oxide Cycloaddition and Tetrasubstituted Olefin Synthesis
Abstract
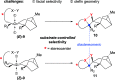
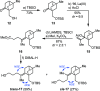
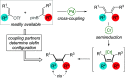
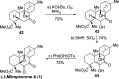
A highly enantioselective and diastereoselective total synthesis of the diterpenoid (-)-mitrephorone A is presented. Key to the synthesis are stereocontrolled 1,4-semihydrogenation of a 1,3-diene to a tetrasubstituted double bond, enzyme-catalyzed malonate desymmetrization, and highly diastereoselective nitrile oxide cycloaddition. The streamlined strategy is a considerable improvement to those reported earlier in terms of diastereo- and enantioselectivity. For the first time, the combination of modern Pd-cross-coupling with Cr-catalyzed reduction allows for rapid access to tetrasubstituted olefins with full stereocontrol.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Fraga B. M. The Trachylobane Diterpenes. Phytochem. Anal. 1994, 5, 49–56. 10.1002/pca.2800050202. - DOI
-
- Li C.; Lee D.; Graf T. N.; Phifer S. S.; Nakanishi Y.; Burgess J. P.; Riswan S.; Setyowati F. M.; Saribi A. M.; Soejarto D. D.; Farnsworth N. R.; Falkinham J. O. III; Kroll D. J.; Kinghorn A. D.; Wani M. C.; Oberlies N. H. A Hexacyclic ent-Trachylobane Diterpenoid Possessing an Oxetane Ring from Mitrephora glabra. Org. Lett. 2005, 7, 5709–5712. 10.1021/ol052498l. - DOI - PMC - PubMed
-
- Wein L. A.; Wurst K.; Angyal P.; Weisheit L.; Magauer T. Synthesis of (−)-Mitrephorone A via a Bioinspired Late Stage C-H Oxidation of (−)-Mitrephorone B. J. Am. Chem. Soc. 2019, 141, 19589–19593. 10.1021/jacs.9b11646. - DOI - PMC - PubMed
-
Very recently, Renata and co-workers reported a semisynthetic approach to (−)-mitrephorone A:
- Zhang X.; King-Smith E.; Dong L.-B.; Yang L.-C.; Rudolf J. D.; Shen B.; Renata H. Divergent synthesis of complex diterpenes through a hybrid oxidative approach. Science 2020, 369, 799–806. 10.1126/science.abb8271. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

