A C. elegans model for neurodegeneration in Cockayne syndrome
- PMID: 33021672
- PMCID: PMC7641758
- DOI: 10.1093/nar/gkaa795
A C. elegans model for neurodegeneration in Cockayne syndrome
Abstract
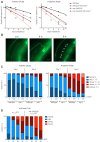
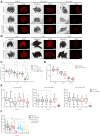
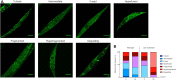
Cockayne syndrome (CS) is a congenital syndrome characterized by growth and mental retardation, and premature ageing. The complexity of CS and mammalian models warrants simpler metazoan models that display CS-like phenotypes that could be studied in the context of a live organism. Here, we provide a characterization of neuronal and mitochondrial aberrations caused by a mutation in the csb-1 gene in Caenorhabditis elegans. We report a progressive neurodegeneration in adult animals that is enhanced upon UV-induced DNA damage. The csb-1 mutants show dysfunctional hyperfused mitochondria that degrade upon DNA damage, resulting in diminished respiratory activity. Our data support the role of endogenous DNA damage as a driving factor of CS-related neuropathology and underline the role of mitochondrial dysfunction in the disease.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- van der Horst G.T., Meira L., Gorgels T.G., de Wit J., Velasco-Miguel S., Richardson J.A., Kamp Y., Vreeswijk M.P., Smit B., Bootsma D. et al. .. UVB radiation-induced cancer predisposition in Cockayne syndrome group A (Csa) mutant mice. DNA Repair (Amst.). 2002; 1:143–157. - PubMed
-
- Nouspikel T. Nucleotide excision repair and neurological diseases. DNA Repair (Amst.). 2008; 7:1155–1167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

