LncRNA VINAS regulates atherosclerosis by modulating NF-κB and MAPK signaling
- PMID: 33021969
- PMCID: PMC7710319
- DOI: 10.1172/jci.insight.140627
LncRNA VINAS regulates atherosclerosis by modulating NF-κB and MAPK signaling
Abstract
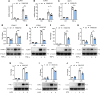
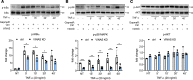
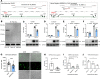
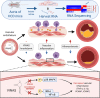
Long noncoding RNAs (lncRNAs) play important roles in regulating diverse cellular processes in the vessel wall, including atherosclerosis. RNA-Seq profiling of intimal lesions revealed a lncRNA, VINAS (Vascular INflammation and Atherosclerosis lncRNA Sequence), that is enriched in the aortic intima and regulates vascular inflammation. Aortic intimal expression of VINAS fell with atherosclerotic progression and rose with regression. VINAS knockdown reduced atherosclerotic lesion formation by 55% in LDL receptor-deficient (LDLR-/-) mice, independent of effects on circulating lipids, by decreasing inflammation in the vessel wall. Loss- and gain-of-function studies in vitro demonstrated that VINAS serves as a critical regulator of inflammation by modulating NF-κB and MAPK signaling pathways. VINAS knockdown decreased the expression of key inflammatory markers, such as MCP-1, TNF-α, IL-1β, and COX-2, in endothelial cells (ECs), vascular smooth muscle cells, and bone marrow-derived macrophages. Moreover, VINAS silencing decreased expression of leukocyte adhesion molecules VCAM-1, E-selectin, and ICAM-1 and reduced monocyte adhesion to ECs. DEP domain containing 4 (DEPDC4), an evolutionary conserved human ortholog of VINAS with approximately 74% homology, showed similar regulation in human and pig atherosclerotic specimens. DEPDC4 knockdown replicated antiinflammatory effects of VINAS in human ECs. These findings reveal a potentially novel lncRNA that regulates vascular inflammation, with broad implications for vascular diseases.
Keywords: Atherosclerosis; Noncoding RNAs; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures



References
-
- Virchow R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI--Atheromatous affection of arteries. 1858. Nutr Rev. 1989;47(1):23–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

