Characteristics of academic publications, preprints, and registered clinical trials on the COVID-19 pandemic
- PMID: 33022014
- PMCID: PMC7537872
- DOI: 10.1371/journal.pone.0240123
Characteristics of academic publications, preprints, and registered clinical trials on the COVID-19 pandemic
Abstract
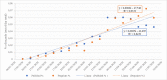
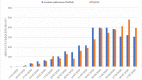
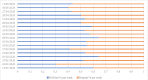
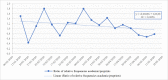
The COVID-19 pandemic has unleashed a deluge of publications. For this cross-sectional study we compared the amount and reporting characteristics of COVID-19-related academic articles and preprints and the number of ongoing clinical trials and systematic reviews. To do this, we searched the PubMed database of citations and abstracts for published life science journals by using appropriate combinations of medical subject headings (MeSH terms), and the COVID-19 section of the MedRxiv and BioRxiv archives up to 20 May 2020 (21 weeks). In addition, we searched Clinicaltrial.gov, Chinese Clinical Trial Registry, EU Clinical Trials Register, and 15 other trial registers, as well as PROSPERO, the international prospective register of systematic reviews. The characteristics of each publication were extracted. Regression analyses and Z tests were used to detect publication trends and their relative proportions. A total of 3635 academic publications and 3805 preprints were retrieved. Only 8.6% (n = 329) of the preprints were already published in indexed journals. The number of academic and preprint publications increased significantly over time (p<0.001). Case reports (6% academic vs 0.9% preprints; p<0.001) and letters (17.4% academic vs 0.5% preprints; p<0.001) accounted for a greater share of academic compared to preprint publications. Differently, randomized controlled trials (0.22% vs 0.63%; p<0.001) and systematic reviews (0.08% vs 5%) made up a greater share of the preprints. The relative proportion of clinical studies registered at Clinicaltrials.gov, Chinese Clinical Trial Registry, and EU Clinical Trials Register was 57.9%, 49.5%, and 98.9%, respectively, most of which were still "recruiting". PROSPERO listed 962 systematic review protocols. Preprints were slightly more prevalent than academic articles but both were increasing in number. The void left by the lack of primary studies was filled by an outpour of immediate opinions (i.e., letters to the editor) published in PubMed-indexed journals. Summarizing, preprints have gained traction as a publishing response to the demand for prompt access to empirical, albeit not peer-reviewed, findings during the present pandemic.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

