Structural Variants Create New Topological-Associated Domains and Ectopic Retinal Enhancer-Gene Contact in Dominant Retinitis Pigmentosa
- PMID: 33022222
- PMCID: PMC7675008
- DOI: 10.1016/j.ajhg.2020.09.002
Structural Variants Create New Topological-Associated Domains and Ectopic Retinal Enhancer-Gene Contact in Dominant Retinitis Pigmentosa
Abstract
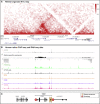
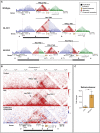
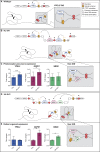
The cause of autosomal-dominant retinitis pigmentosa (adRP), which leads to loss of vision and blindness, was investigated in families lacking a molecular diagnosis. A refined locus for adRP on Chr17q22 (RP17) was delineated through genotyping and genome sequencing, leading to the identification of structural variants (SVs) that segregate with disease. Eight different complex SVs were characterized in 22 adRP-affected families with >300 affected individuals. All RP17 SVs had breakpoints within a genomic region spanning YPEL2 to LINC01476. To investigate the mechanism of disease, we reprogrammed fibroblasts from affected individuals and controls into induced pluripotent stem cells (iPSCs) and differentiated them into photoreceptor precursor cells (PPCs) or retinal organoids (ROs). Hi-C was performed on ROs, and differential expression of regional genes and a retinal enhancer RNA at this locus was assessed by qPCR. The epigenetic landscape of the region, and Hi-C RO data, showed that YPEL2 sits within its own topologically associating domain (TAD), rich in enhancers with binding sites for retinal transcription factors. The Hi-C map of RP17 ROs revealed creation of a neo-TAD with ectopic contacts between GDPD1 and retinal enhancers, and modeling of all RP17 SVs was consistent with neo-TADs leading to ectopic retinal-specific enhancer-GDPD1 accessibility. qPCR confirmed increased expression of GDPD1 and increased expression of the retinal enhancer that enters the neo-TAD. Altered TAD structure resulting in increased retinal expression of GDPD1 is the likely convergent mechanism of disease, consistent with a dominant gain of function. Our study highlights the importance of SVs as a genomic mechanism in unsolved Mendelian diseases.
Keywords: GDPD; Hi-C; RP17; dominant retinitis pigmentosa; ectopic expression; photoreceptor precursors cells; retinal organoids; stem cells; structural variants; topologically associated domains; whole-genome sequencing.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Verbakel S.K., van Huet R.A.C., Boon C.J.F., den Hollander A.I., Collin R.W.J., Klaver C.C.W., Hoyng C.B., Roepman R., Klevering B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018;66:157–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

