Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities
- PMID: 33022379
- PMCID: PMC10368393
- DOI: 10.1016/j.preteyeres.2020.100906
Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities
Abstract
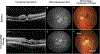
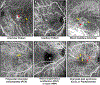
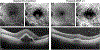
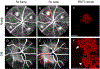
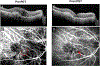
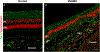
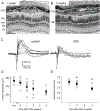
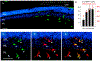
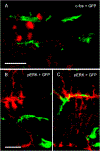
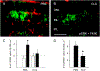
Intravitreal anti-vascular endothelial growth factor (VEGF) drugs have revolutionized the treatment of neovascular age-related macular degeneration (NVAMD). However, many patients suffer from incomplete response to anti-VEGF therapy (IRT), which is defined as (1) persistent (plasma) fluid exudation; (2) unresolved or new hemorrhage; (3) progressive lesion fibrosis; and/or (4) suboptimal vision recovery. The first three of these collectively comprise the problem of persistent disease activity (PDA) in spite of anti-VEGF therapy. Meanwhile, the problem of suboptimal vision recovery (SVR) is defined as a failure to achieve excellent functional visual acuity of 20/40 or better in spite of sufficient anti-VEGF treatment. Thus, incomplete response to anti-VEGF therapy, and specifically PDA and SVR, represent significant clinical unmet needs. In this review, we will explore PDA and SVR in NVAMD, characterizing the clinical manifestations and exploring the pathobiology of each. We will demonstrate that PDA occurs most frequently in NVAMD patients who develop high-flow CNV lesions with arteriolarization, in contrast to patients with capillary CNV who are highly responsive to anti-VEGF therapy. We will review investigations of experimental CNV and demonstrate that both types of CNV can be modeled in mice. We will present and consider a provocative hypothesis: formation of arteriolar CNV occurs via a distinct pathobiology, termed neovascular remodeling (NVR), wherein blood-derived macrophages infiltrate the incipient CNV lesion, recruit bone marrow-derived mesenchymal precursor cells (MPCs) from the circulation, and activate MPCs to become vascular smooth muscle cells (VSMCs) and myofibroblasts, driving the development of high-flow CNV with arteriolarization and perivascular fibrosis. In considering SVR, we will discuss the concept that limited or poor vision in spite of anti-VEGF may not be caused simply by photoreceptor degeneration but instead may be associated with photoreceptor synaptic dysfunction in the neurosensory retina overlying CNV, triggered by infiltrating blood-derived macrophages and mediated by Müller cell activation Finally, for each of PDA and SVR, we will discuss current approaches to disease management and treatment and consider novel avenues for potential future therapies.
Keywords: Anti-VEGF; Anti-VEGF resistance; Choroidal neovascularization; Macrophage; Mesenchymal precursor cell; Monocyte; Müller cell; Neovascular age-related macular degeneration; Neovascular remodeling; Persistent disease activity; Photoreceptor synaptic dysfunction; Suboptimal vision recovery.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures



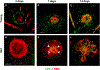






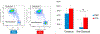

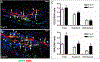








References
-
- Al-Khersan H, Hussain RM, Ciulla TA, Dugel PU, 2019. ’Innovative therapies for neovascular age-related macular degeneration. Expet Opin. Pharmacother. 20, 1879–1891. - PubMed
-
- Al-Sheikh M, Iafe NA, Phasukkijwatana N, Sadda SR, Sarraf D, 2018. Biomarkers of neovascular activity in age-related macular degeneration using optical coherence tomography angiography’. Retina 38, 220–230. - PubMed
-
- Allingham MJ, Tserentsoodol N, Saloupis P, Mettu PS, Cousins SW, 2018. ’Aldosterone exposure causes increased retinal edema and severe retinopathy following laser-induced retinal vein occlusion in mice. Invest. Ophthalmol. Vis. Sci. 59, 3355–3365. - PubMed
-
- Allingham MJ, Mettu PS, Cousins SW, 2019. ’Elamipretide, a mitochondrial- targeted drug, for the treatment of vision loss in dry AMD with high risk drusen: results of the phase 1 ReCLAIM study. Invest. Ophthalmol. Vis. Sci. 60, 361–61.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

