"When Offered to Participate": A Systematic Review and Meta-Analysis of Patient Agreement to Participate in Cancer Clinical Trials
- PMID: 33022716
- PMCID: PMC7936064
- DOI: 10.1093/jnci/djaa155
"When Offered to Participate": A Systematic Review and Meta-Analysis of Patient Agreement to Participate in Cancer Clinical Trials
Abstract
Background: Patient participation in clinical trials is vital for knowledge advancement and outcomes improvement. Few adult cancer patients participate in trials. Although patient.
decision-making about trial participation has been frequently examined, the participation rate for patients actually offered a trial is unknown.
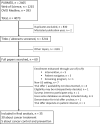
Methods: A systematic review and meta-analysis using 3 major search engines was undertaken. We identified studies from January 1, 2000, to January 1, 2020, that examined clinical trial participation in the United States. Studies must have specified the numbers of patients offered a trial and the number enrolled. A random effects model of proportions was used. All statistical tests were 2-sided.
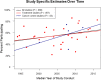
Results: We identified 35 studies (30 about treatment trials and 5 about cancer control trials) among which 9759 patients were offered trial participation. Overall, 55.0% (95% confidence interval [CI] = 49.4% to 60.5%) of patients agreed to enroll. Participation rates did not differ between treatment (55.0%, 95% CI = 48.9% to 60.9%) and cancer control trials (55.3%, 95% CI = 38.9% to 71.1%; P = .98). Black patients participated at similar rates (58.4%, 95% CI = 46.8% to 69.7%) compared with White patients (55.1%, 95% CI = 44.3% to 65.6%; P = .88). The main reasons for nonparticipation were treatment choice or lack of interest.
Conclusions: More than half of all cancer patients offered a clinical trial do participate. These findings upend several conventional beliefs about cancer clinical trial participation, including that Black patients are less likely to agree to participate and that patient decision-making is the primary barrier to participation. Policies and interventions to improve clinical trial participation should focus more on modifiable systemic structural and clinical barriers, such as improving access to available trials and broadening eligibility criteria.
© The Author(s) 2020. Published by Oxford University Press.
Figures


Comment in
-
Revisiting Barriers to Clinical Trials Accrual.J Natl Cancer Inst. 2021 Mar 1;113(3):219-220. doi: 10.1093/jnci/djaa156. J Natl Cancer Inst. 2021. PMID: 33022708 Free PMC article. No abstract available.
Similar articles
-
Systematic Review and Meta-Analysis of the Magnitude of Structural, Clinical, and Physician and Patient Barriers to Cancer Clinical Trial Participation.J Natl Cancer Inst. 2019 Mar 1;111(3):245-255. doi: 10.1093/jnci/djy221. J Natl Cancer Inst. 2019. PMID: 30856272 Free PMC article.
-
Identifying patient values impacting the decision whether to participate in early phase clinical cancer trials: A systematic review.Cancer Treat Rev. 2021 Jul;98:102217. doi: 10.1016/j.ctrv.2021.102217. Epub 2021 Apr 28. Cancer Treat Rev. 2021. PMID: 33965892
-
Association of Patient Comorbid Conditions With Cancer Clinical Trial Participation.JAMA Oncol. 2019 Mar 1;5(3):326-333. doi: 10.1001/jamaoncol.2018.5953. JAMA Oncol. 2019. PMID: 30629092 Free PMC article.
-
Identifying motivations and barriers to patient participation in clinical trials.J Cancer Educ. 2006 Winter;21(4):237-42. doi: 10.1080/08858190701347838. J Cancer Educ. 2006. PMID: 17542716 Clinical Trial.
-
Discordant attitudes and beliefs about cancer clinical trial participation between physicians, research staff, and cancer patients.Clin Trials. 2020 Apr;17(2):184-194. doi: 10.1177/1740774520901514. Epub 2020 Feb 3. Clin Trials. 2020. PMID: 32009456 Free PMC article.
Cited by
-
The potential clinical utility of Whole Genome Sequencing for patients with cancer: evaluation of a regional implementation of the 100,000 Genomes Project.Br J Cancer. 2024 Dec;131(11):1805-1813. doi: 10.1038/s41416-024-02890-6. Epub 2024 Oct 30. Br J Cancer. 2024. PMID: 39478124 Free PMC article.
-
Clinical effectiveness of vaginal pessary self-management vs clinic-based care for pelvic organ prolapse (TOPSY): a randomised controlled superiority trial.EClinicalMedicine. 2023 Nov 23;66:102326. doi: 10.1016/j.eclinm.2023.102326. eCollection 2023 Dec. EClinicalMedicine. 2023. PMID: 38078194 Free PMC article.
-
Revisiting Barriers to Clinical Trials Accrual.J Natl Cancer Inst. 2021 Mar 1;113(3):219-220. doi: 10.1093/jnci/djaa156. J Natl Cancer Inst. 2021. PMID: 33022708 Free PMC article. No abstract available.
-
Approaches to Clinical Complete Response after Neoadjuvant Chemotherapy in Muscle-Invasive Bladder Cancer: Possibilities and Limitations.Cancers (Basel). 2023 Feb 19;15(4):1323. doi: 10.3390/cancers15041323. Cancers (Basel). 2023. PMID: 36831665 Free PMC article. Review.
-
The emotional side of taking part in a cancer clinical trial.PLoS One. 2023 Apr 24;18(4):e0284268. doi: 10.1371/journal.pone.0284268. eCollection 2023. PLoS One. 2023. PMID: 37093865 Free PMC article.
References
-
- Institute of Medicine. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington, DC: National Academies Press; 2010. - PubMed
-
- Murthy VH, Krumholz HM, Gross CP.. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. - PubMed
-
- Sateren WB, Trimble EL, Abrams J, et al.How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20(8):2109–2117. - PubMed
-
- Tejeda HA, Green SB, Trimble EL, et al.Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88(12):812–816. - PubMed

