Covid-19 and kidney injury: Pathophysiology and molecular mechanisms
- PMID: 33022818
- PMCID: PMC7646060
- DOI: 10.1002/rmv.2176
Covid-19 and kidney injury: Pathophysiology and molecular mechanisms
Abstract
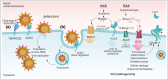
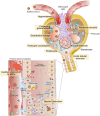
The novel coronavirus (SARS-CoV-2) has turned into a life-threatening pandemic disease (Covid-19). About 5% of patients with Covid-19 have severe symptoms including septic shock, acute respiratory distress syndrome, and the failure of several organs, while most of them have mild symptoms. Frequently, the kidneys are involved through direct or indirect mechanisms. Kidney involvement mainly manifests itself as proteinuria and acute kidney injury (AKI). The SARS-CoV-2-induced kidney damage is expected to be multifactorial; directly it can infect the kidney podocytes and proximal tubular cells and based on an angiotensin-converting enzyme 2 (ACE2) pathway it can lead to acute tubular necrosis, protein leakage in Bowman's capsule, collapsing glomerulopathy and mitochondrial impairment. The SARS-CoV-2-driven dysregulation of the immune responses including cytokine storm, macrophage activation syndrome, and lymphopenia can be other causes of the AKI. Organ interactions, endothelial dysfunction, hypercoagulability, rhabdomyolysis, and sepsis are other potential mechanisms of AKI. Moreover, lower oxygen delivery to kidney may cause an ischaemic injury. Understanding the fundamental molecular pathways and pathophysiology of kidney injury and AKI in Covid-19 is necessary to develop management strategies and design effective therapies.
Keywords: SARS-CoV-2; acute kidney injury; angiotensin; bardikinin; coronovirus; proteinuria; renal injury.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
No conflict of interest is declared.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

