Impact of tumor volume enlargement after induction chemotherapy on subsequent radiotherapy in locally advanced nasopharyngeal carcinoma: A propensity-score matching analysis
- PMID: 33022902
- PMCID: PMC7724294
- DOI: 10.1002/cam4.3494
Impact of tumor volume enlargement after induction chemotherapy on subsequent radiotherapy in locally advanced nasopharyngeal carcinoma: A propensity-score matching analysis
Abstract
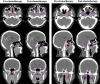
A small proportion of nasopharyngeal carcinoma (NPC) patients show resistance to induction chemotherapy (IC). This study sought to investigate the impact of tumor volume enlargement after IC on the dosimetric parameters of subsequent radiotherapy. The records of a total of 240 locally advanced NPC patients who received IC followed by concurrent chemoradiotherapy were retrospectively reviewed. Patients with a tumor volume enlargement of ≥10% and patients with a tumor volume reduction of ≥10% after induction chemotherapy were classified as the enlargement group and the control group, respectively. The dosimetric parameters of the planning target volumes (PTVs) and the organs at risk (OARs) were compared between the matched groups after propensity score matching (PSM). For the gross tumor volume of nasopharynx (GTVnx), 21 patients and 127 patients were classified as the enlargement group and the control group, respectively. After matching, 20 sub-pairs of 40 patients were generated in the post-PSM cohort. The GTVnx enlargement group exhibited no significant disadvantages in all of the dosimetric parameters, except in the planning organ-at-risk volume (PRV) of contralateral lens (Dmax, 722 cGy vs. 634 cGy, p = 0.041). For the gross tumor volume of lymph nodes (GTVnd), 44 patients and 144 patients were classified as the enlargement group and the control group, respectively. After matching, 39 sub-pairs of 78 patients were generated in the post-PSM cohort. The GTVnd enlargement group exhibited no significant disadvantages in all of the dosimetric parameters. Univariate and multivariate analyses showed that the enlargement of GTVnx and the enlargement of GTVnd were not independently associated with any of the dosimetric parameters. A tumor volume enlargement of ≥10% in GTVnx or GTVnd after induction chemotherapy has no significant impact on the dosimetric parameters of subsequent radiotherapy in locally advanced NPC.
Keywords: dosimetry; induction chemotherapy; nasopharyngeal carcinoma; propensity score matching; tumor volume.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Gemcitabine Versus Docetaxel Plus Cisplatin as Induction Chemotherapy in Nasopharyngeal Carcinoma.Laryngoscope. 2022 Dec;132(12):2379-2387. doi: 10.1002/lary.30092. Epub 2022 Mar 3. Laryngoscope. 2022. PMID: 35238403
-
Locoregional Control and Mild Late Toxicity After Reducing Target Volumes and Radiation Doses in Patients With Locoregionally Advanced Nasopharyngeal Carcinoma Treated With Induction Chemotherapy (IC) Followed by Concurrent Chemoradiotherapy: 10-Year Results of a Phase 2 Study.Int J Radiat Oncol Biol Phys. 2019 Jul 15;104(4):836-844. doi: 10.1016/j.ijrobp.2019.03.043. Epub 2019 Apr 5. Int J Radiat Oncol Biol Phys. 2019. PMID: 30954521 Clinical Trial.
-
The change in tumor volume after induction chemotherapy with docetaxel plus cisplatin in 259 nasopharyngeal carcinoma patients.Eur Arch Otorhinolaryngol. 2021 Aug;278(8):3027-3035. doi: 10.1007/s00405-020-06477-8. Epub 2021 Jan 2. Eur Arch Otorhinolaryngol. 2021. PMID: 33386968
-
Concurrent chemoradiotherapy with additional chemotherapy for nasopharyngeal carcinoma: A pooled analysis of propensity score-matching studies.Head Neck. 2021 Jun;43(6):1912-1927. doi: 10.1002/hed.26664. Epub 2021 Feb 28. Head Neck. 2021. PMID: 33644916 Review.
-
Induction chemotherapy for locally advanced nasopharyngeal carcinoma treated with concurrent chemoradiation: A systematic review and meta-analysis.Radiother Oncol. 2018 Oct;129(1):10-17. doi: 10.1016/j.radonc.2018.02.027. Epub 2018 Mar 16. Radiother Oncol. 2018. PMID: 29555182
Cited by
-
Plasma Metabolic Profiles-Based Prediction of Induction Chemotherapy Efficacy in Nasopharyngeal Carcinoma: Results of a Bidirectional Clinical Trial.Clin Cancer Res. 2024 Jul 15;30(14):2925-2936. doi: 10.1158/1078-0432.CCR-23-3608. Clin Cancer Res. 2024. PMID: 38713248 Free PMC article. Clinical Trial.
References
-
- Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prevention. 2006;15(10):1765–1777. - PubMed
-
- Lee HM, Okuda KS, Gonzalez FE, et al. Current perspectives on nasopharyngeal carcinoma. Adv Exp Med Biol. 2019;1164:11–34. - PubMed
-
- Cao SM, Yang Q, Guo L, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14–23. - PubMed
-
- Hong RL, Hsiao CF, Ting LL, et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma‐Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Annals Oncol. 2018;29(9):1972–1979. - PubMed
-
- Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin‐radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27(2):242–249. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

