A Perspective on Therapeutic Pan-Resistance in Metastatic Cancer
- PMID: 33022920
- PMCID: PMC7582598
- DOI: 10.3390/ijms21197304
A Perspective on Therapeutic Pan-Resistance in Metastatic Cancer
Abstract
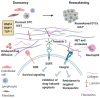
Metastatic spread represents the leading cause of disease-related mortality among cancer patients. Many cancer patients suffer from metastatic relapse years or even decades after radical surgery for the primary tumor. This clinical phenomenon is explained by the early dissemination of cancer cells followed by a long period of dormancy. Although dormancy could be viewed as a window of opportunity for therapeutic interventions, dormant disseminated cancer cells and micrometastases, as well as emergent outgrowing macrometastases, exhibit a generalized, innate resistance to chemotherapy and even immunotherapy. This therapeutic pan-resistance, on top of other adaptive responses to targeted agents such as acquired mutations and lineage plasticity, underpins the current difficulties in eradicating cancer. In the present review, we attempt to provide a framework to understand the underlying biology of this major issue.
Keywords: disseminated tumor cells; dormancy; e-cadherin; epigenetics; immune checkpoint blockade; metabolic plasticity; metastasis; metastatic microenvironment; therapy resistance.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


References
-
- Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni L., Valagussa P., et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. - DOI - PubMed
-
- Asselain B., Barlow W., Bartlett J., Bergh J., Bergsten-Nordström E., Bliss J., Boccardo F., Boddington C., Bogaerts J., Bonadonna G., et al. Early Breast Cancer Trialists’ Collaborative G. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39. doi: 10.1016/S1470-2045(17)30777-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

