Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities
- PMID: 33022970
- PMCID: PMC7582585
- DOI: 10.3390/molecules25194537
Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities
Abstract
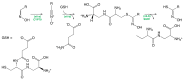
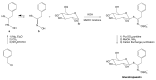
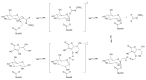
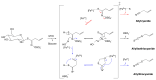
Glucosinolates (GSLs) are secondary plant metabolites abundantly found in plant order Brassicales. GSLs are constituted by an S-β-d-glucopyrano unit anomerically connected to O-sulfated (Z)-thiohydroximate moiety. The side-chain of the O-sulfate thiohydroximate moiety, which is derived from a different amino acid, contributes to the diversity of natural GSL, with more than 130 structures identified and validated to this day. Both the structural diversity of GSL and their biological implication in plants have been biochemically studied. Although chemical syntheses of GSL have been devised to give access to these secondary metabolites, direct extraction from biomass remains the conventional method to isolate natural GSL. While intact GSLs are biologically inactive, various products, including isothiocyanates, nitriles, epithionitriles, and cyanides obtained through their hydrolysis of GSLs, exhibit many different biological activities, among which several therapeutic benefits have been suggested. This article reviews natural occurrence, accessibility via chemical, synthetic biochemical pathways of GSL, and the current methodology of extraction, purification, and characterization. Structural information, including the most recent classification of GSL, and their stability and storage conditions will also be discussed. The biological perspective will also be explored to demonstrate the importance of these prominent metabolites.
Keywords: Brassicaceae family; Brassicales; Moringacea family; glucosinolates; myrosinases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
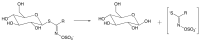

References
-
- Borek V., Morra M.J., Brown P.D., McCaffrey J.P. Allelochemicals Produced during Sinigrin Decomposition in Soil. J. Agric. Food Chem. 1994;42:1030–1034. doi: 10.1021/jf00040a037. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

