Synergism of the Combination of Traditional Antibiotics and Novel Phenolic Compounds against Escherichia coli
- PMID: 33023003
- PMCID: PMC7600547
- DOI: 10.3390/pathogens9100811
Synergism of the Combination of Traditional Antibiotics and Novel Phenolic Compounds against Escherichia coli
Abstract
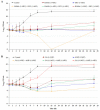
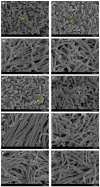
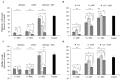
Pathogenic Escherichia coli (E. coli)-associated infections are becoming difficult to treat because of the rapid emergence of antibiotic-resistant strains. Novel approaches are required to prevent the progression of resistance and to extend the lifespan of existing antibiotics. This study was designed to improve the effectiveness of traditional antibiotics against E. coli using a combination of the gallic acid (GA), hamamelitannin, epicatechin gallate, epigallocatechin, and epicatechin. The fractional inhibitory concentration index (FICI) of each of the phenolic compound-antibiotic combinations against E. coli was ascertained. Considering the clinical significance and FICI, two combinations (hamamelitannin-erythromycin and GA-ampicillin) were evaluated for their impact on certain virulence factors of E. coli. Finally, the effects of hamamelitannin and GA on Rattus norvegicus (IEC-6) cell viability were investigated. The FICIs of the antibacterial combinations against E. coli were 0.281-1.008. The GA-ampicillin and hamamelitannin-erythromycin combinations more effectively prohibited the growth, biofilm viability, and swim and swarm motilities of E. coli than individual antibiotics. The concentration of hamamelitannin and GA required to reduce viability by 50% (IC50) in IEC-6 cells was 988.54 μM and 564.55 μM, correspondingly. GA-ampicillin and hamamelitannin-erythromycin may be potent combinations and promising candidates for eradicating pathogenic E. coli in humans and animals.
Keywords: ampicillin; antibacterial agents; bacterial pathogenicity; erythromycin; synergistic effect.
Conflict of interest statement
None of the authors have any conflicts of interest to declare.
Figures




Similar articles
-
A pharmacodynamic investigation to assess the synergism of orbifloxacin and propyl gallate against Escherichia coli.Front Pharmacol. 2022 Sep 15;13:989395. doi: 10.3389/fphar.2022.989395. eCollection 2022. Front Pharmacol. 2022. PMID: 36188537 Free PMC article.
-
Advances and mechanisms of traditional Chinese medicine and its active ingredients against antibiotic-resistant Escherichia coli infections.J Pharm Anal. 2025 Feb;15(2):101117. doi: 10.1016/j.jpha.2024.101117. Epub 2024 Oct 2. J Pharm Anal. 2025. PMID: 40026356 Free PMC article. Review.
-
In vitro synergistic potentials of novel antibacterial combination therapies against Salmonella enterica serovar Typhimurium.BMC Microbiol. 2020 May 14;20(1):118. doi: 10.1186/s12866-020-01810-x. BMC Microbiol. 2020. PMID: 32410630 Free PMC article.
-
Synergistic Antimicrobial Effect of Antimicrobial Peptides CATH-1, CATH-3, and PMAP-36 With Erythromycin Against Bacterial Pathogens.Front Microbiol. 2022 Jul 15;13:953720. doi: 10.3389/fmicb.2022.953720. eCollection 2022. Front Microbiol. 2022. PMID: 35910608 Free PMC article.
-
General Overview of Phenolics from Plant to Laboratory, Good Antibacterials or Not.Pharmacogn Rev. 2017 Jul-Dec;11(22):123-127. doi: 10.4103/phrev.phrev_43_16. Pharmacogn Rev. 2017. PMID: 28989246 Free PMC article. Review.
Cited by
-
Synergistic Activity of Equol and Meropenem against Carbapenem-Resistant Escherichia coli.Antibiotics (Basel). 2021 Feb 5;10(2):161. doi: 10.3390/antibiotics10020161. Antibiotics (Basel). 2021. PMID: 33562526 Free PMC article.
-
A pharmacodynamic investigation to assess the synergism of orbifloxacin and propyl gallate against Escherichia coli.Front Pharmacol. 2022 Sep 15;13:989395. doi: 10.3389/fphar.2022.989395. eCollection 2022. Front Pharmacol. 2022. PMID: 36188537 Free PMC article.
-
Identified Seaweed Compound Diphenylmethane Serves as an Efflux Pump Inhibitor in Drug-Resistant Escherichia coli.Antibiotics (Basel). 2021 Nov 10;10(11):1378. doi: 10.3390/antibiotics10111378. Antibiotics (Basel). 2021. PMID: 34827316 Free PMC article.
-
Phenolic Compounds from Pyrus communis Residues: Mechanisms of Antibacterial Action and Therapeutic Applications.Antibiotics (Basel). 2025 Mar 8;14(3):280. doi: 10.3390/antibiotics14030280. Antibiotics (Basel). 2025. PMID: 40149091 Free PMC article. Review.
-
Advances and mechanisms of traditional Chinese medicine and its active ingredients against antibiotic-resistant Escherichia coli infections.J Pharm Anal. 2025 Feb;15(2):101117. doi: 10.1016/j.jpha.2024.101117. Epub 2024 Oct 2. J Pharm Anal. 2025. PMID: 40026356 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

