TAZ Represses the Neuronal Commitment of Neural Stem Cells
- PMID: 33023162
- PMCID: PMC7600930
- DOI: 10.3390/cells9102230
TAZ Represses the Neuronal Commitment of Neural Stem Cells
Abstract
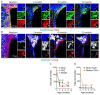
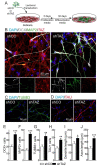
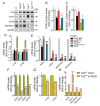
The mechanisms involved in regulation of quiescence, proliferation, and reprogramming of Neural Stem Progenitor Cells (NSPCs) of the mammalian brain are still poorly defined. Here, we studied the role of the transcriptional co-factor TAZ, regulated by the WNT and Hippo pathways, in the homeostasis of NSPCs. We found that, in the murine neurogenic niches of the striatal subventricular zone and the dentate gyrus granular zone, TAZ is highly expressed in NSPCs and declines with ageing. Moreover, TAZ expression is lost in immature neurons of both neurogenic regions. To characterize mechanistically the role of TAZ in neuronal differentiation, we used the midbrain-derived NSPC line ReNcell VM to replicate in a non-animal model the factors influencing NSPC differentiation to the neuronal lineage. TAZ knock-down and forced expression in NSPCs led to increased and reduced neuronal differentiation, respectively. TEADs-knockdown indicated that these TAZ co-partners are required for the suppression of NSPCs commitment to neuronal differentiation. Genetic manipulation of the TAZ/TEAD system showed its participation in transcriptional repression of SOX2 and the proneuronal genes ASCL1, NEUROG2, and NEUROD1, leading to impediment of neurogenesis. TAZ is usually considered a transcriptional co-activator promoting stem cell proliferation, but our study indicates an additional function as a repressor of neuronal differentiation.
Keywords: ASCL; NEUROD1; NEUROG2; SOX2; hippo; neural stem cells; neurogenesis; neuronal differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Activation of metabotropic glutamate receptor 4 regulates proliferation and neural differentiation in neural stem/progenitor cells of the rat subventricular zone and increases phosphatase and tensin homolog protein expression.J Neurochem. 2021 Feb;156(4):465-480. doi: 10.1111/jnc.14984. Epub 2020 Mar 12. J Neurochem. 2021. PMID: 32052426
-
An easy and cost-effective method for the isolation and culturing of neural stem/progenitor cells from the subventricular (SVZ) and dentate gyrus (DG) of adult mouse brain.J Neurosci Methods. 2024 Apr;404:110060. doi: 10.1016/j.jneumeth.2024.110060. Epub 2024 Jan 19. J Neurosci Methods. 2024. PMID: 38244848
-
A Role for Nrf2 Expression in Defining the Aging of Hippocampal Neural Stem Cells.Cell Transplant. 2018 Apr;27(4):589-606. doi: 10.1177/0963689718774030. Epub 2018 Jun 5. Cell Transplant. 2018. PMID: 29871525 Free PMC article.
-
Transcriptional regulation of adult neural stem/progenitor cells: tales from the subventricular zone.Neural Regen Res. 2020 Oct;15(10):1773-1783. doi: 10.4103/1673-5374.280301. Neural Regen Res. 2020. PMID: 32246617 Free PMC article. Review.
-
Metabolism and neurogenesis.Curr Opin Neurobiol. 2017 Feb;42:45-52. doi: 10.1016/j.conb.2016.11.006. Epub 2016 Dec 1. Curr Opin Neurobiol. 2017. PMID: 27915086 Review.
Cited by
-
An Example of Neuro-Glial Commitment and Differentiation of Muse Stem Cells Obtained from Patients with IQSEC2-Related Neural Disorder: A Possible New Cell-Based Disease Model.Cells. 2023 Mar 23;12(7):977. doi: 10.3390/cells12070977. Cells. 2023. PMID: 37048050 Free PMC article.
-
Herpes Simplex Virus Type 1 Induces AD-like Neurodegeneration Markers in Human Progenitor and Differentiated ReNcell VM Cells.Microorganisms. 2023 May 4;11(5):1205. doi: 10.3390/microorganisms11051205. Microorganisms. 2023. PMID: 37317179 Free PMC article.
-
YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches.Nat Cancer. 2023 Jan;4(1):9-26. doi: 10.1038/s43018-022-00473-z. Epub 2022 Dec 23. Nat Cancer. 2023. PMID: 36564601 Free PMC article. Review.
-
Combination of dasatinib and quercetin promotes osteogenic differentiation and stemness maintenance of hPDLSCs via YAP/TAZ.Anim Cells Syst (Seoul). 2025 Mar 12;29(1):19-29. doi: 10.1080/19768354.2025.2477050. eCollection 2025. Anim Cells Syst (Seoul). 2025. PMID: 40098711 Free PMC article.
-
Cell-Cell Contact Mediates Gene Expression and Fate Choice of Human Neural Stem/Progenitor Cells.Cells. 2022 May 25;11(11):1741. doi: 10.3390/cells11111741. Cells. 2022. PMID: 35681435 Free PMC article.
References
-
- Moreno-Jimenez E.P., Flor-Garcia M., Terreros-Roncal J., Rabano A., Cafini F., Pallas-Bazarra N., Avila J., Llorens-Martin M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. - DOI - PubMed
-
- Marchetti B., Tirolo C., L’Episcopo F., Caniglia S., Testa N., Smith J.A., Pluchino S., Serapide M.F. Parkinson’s disease, aging and adult neurogenesis: Wnt/beta-catenin signalling as the key to unlock the mystery of endogenous brain repair. Aging Cell. 2020;19:e13101. doi: 10.1111/acel.13101. - DOI - PMC - PubMed

