High Titer Persistent Neutralizing Antibodies Induced by TSST-1 Variant Vaccine Against Toxic Shock Cytokine Storm
- PMID: 33023185
- PMCID: PMC7601046
- DOI: 10.3390/toxins12100640
High Titer Persistent Neutralizing Antibodies Induced by TSST-1 Variant Vaccine Against Toxic Shock Cytokine Storm
Abstract
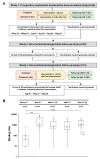
Staphylococcal superantigen toxins lead to a devastating cytokine storm resulting in shock and multi-organ failure. We have previously assessed the safety and immunogenicity of a recombinant toxic shock syndrome toxin 1 variant vaccine (rTSST-1v) in clinical trials (NCT02971670 and NCT02340338). The current study assessed neutralizing antibody titers after repeated vaccination with escalating doses of rTSST-1v. At study entry, 23 out of 34 subjects (67.6%) had neutralizing antibody titers inhibiting T cell activation as determined by 3H-thymidine incorporation at a serum dilution of ≤1:100 with similar figures for inhibition of IL-2 activation (19 of 34 subjects, 55.9%) as assessed by quantitative PCR. After the first vaccination, numbers of subjects with neutralization titers inhibiting T cell activation (61.7% ≥ 1:1000) and inhibiting IL-2 gene induction (88.2% ≥ 1:1000) increased. The immune response was augmented after the second vaccination (inhibiting T cell activation: 78.8% ≥ 1:1000; inhibiting IL-2 induction: 93.9% ≥ 1:1000) corroborated with a third immunization months later in a small subgroup of subjects. Assessment of IFNγ, TNFα and IL-6 inhibition revealed similar results, whereas neutralization titers did not change in placebo participants. Antibody titer studies show that vaccination with rTSST-1v in subjects with no/low neutralizing antibodies can rapidly induce high titer neutralizing antibodies persisting over months.
Keywords: Staphylococcus; immunogenicity; neutralizing antibodies; toxic shock syndrome.
Conflict of interest statement
M.S., C.F. and B.J. declare no competing interests. M.M.E. is the CEO of the study sponsor Biomedizinische Forschung & Bio-Produkte AG, a biotechnology company engaged in the development of BioMed rTSST-1 variant vaccine. A.R., N.S., and N.M. are employees of the study sponsor Biomedizinische Forschung & Bio-Produkte AG. M.M.E. is head of the immunological laboratory, and A.R., N.S., and N.M. are co-workers.
Figures


References
-
- Abraham J., Mansour C., Veledar E., Khan B., Lerakis S. Staphylococcus aureus bacteremia and endocarditis: The Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am. Heart J. 2004;147:536–539. doi: 10.1016/j.ahj.2003.09.018. - DOI - PubMed
-
- Chung J.W., Greenwood-Quaintance K.E., Karau M.J., Tilahun A., Khaleghi S.R., Chowdhary V.R., David C.S., Patel R., Rajagopalan G. Superantigens produced by catheter-associated Staphylococcus aureus elicit systemic inflammatory disease in the absence of bacteremia. J. Leukoc. Biol. 2015;98:271–281. doi: 10.1189/jlb.4A1214-577RR. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

