Nutrition and microRNAs: Novel Insights to Fight Sarcopenia
- PMID: 33023202
- PMCID: PMC7601022
- DOI: 10.3390/antiox9100951
Nutrition and microRNAs: Novel Insights to Fight Sarcopenia
Abstract
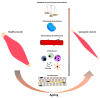
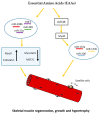
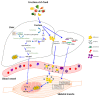
Sarcopenia is a progressive age-related loss of skeletal muscle mass and strength, which may result in increased physical frailty and a higher risk of adverse events. Low-grade systemic inflammation, loss of muscle protein homeostasis, mitochondrial dysfunction, and reduced number and function of satellite cells seem to be the key points for the induction of muscle wasting, contributing to the pathophysiological mechanisms of sarcopenia. While a range of genetic, hormonal, and environmental factors has been reported to contribute to the onset of sarcopenia, dietary interventions targeting protein or antioxidant intake may have a positive effect in increasing muscle mass and strength, regulating protein homeostasis, oxidative reaction, and cell autophagy, thus providing a cellular lifespan extension. MicroRNAs (miRNAs) are endogenous small non-coding RNAs, which control gene expression in different tissues. In skeletal muscle, a range of miRNAs, named myomiRNAs, are involved in many physiological processes, such as growth, development, and maintenance of muscle mass and function. This review aims to present and to discuss some of the most relevant molecular mechanisms related to the pathophysiological effect of sarcopenia. Besides, we explored the role of nutrition as a possible way to counteract the loss of muscle mass and function associated with ageing, with special attention paid to nutrient-dependent miRNAs regulation. This review will provide important information to better understand sarcopenia and, thus, to facilitate research and therapeutic strategies to counteract the pathophysiological effect of ageing.
Keywords: TNF; ageing; autophagy; fructose; hormesis; inflammation; nutrition; oxidative stress; skeletal muscle; uric acid; vitagene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rolland Y., Czerwinski S., Van Kan G.A., Morley J.E., Cesari M., Onder G., Woo J., Baumgartner R., Pillard F., Boirie Y., et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

