Transplantation Efficacy of Human Ciliary Epithelium Cells from Fetal Eye and Lin-ve Stem Cells from Umbilical Cord Blood in the Murine Retinal Degeneration Model of Laser Injury
- PMID: 33023312
- PMCID: PMC7784603
- DOI: 10.1177/0963689720946031
Transplantation Efficacy of Human Ciliary Epithelium Cells from Fetal Eye and Lin-ve Stem Cells from Umbilical Cord Blood in the Murine Retinal Degeneration Model of Laser Injury
Abstract
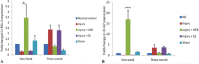
A number of degenerative conditions affecting the neural retina including age-related macular degeneration have no successful treatment, resulting in partial or complete vision loss. There are a number of stem cell replacement strategies for recovery of retinal damage using cells from variable sources. However, literature is still deficit in the comparison of efficacy of types of stem cells. The purpose of the study was to compare the therapeutic efficacy of undifferentiated cells, i.e., lineage negative stem cells (Lin-ve SC) with differentiated neurosphere derived from ciliary epithelium (CE) cells on retinal markers associated with laser-induced retinal injury. Laser-induced photocoagulation was carried out to disrupt Bruch's membrane and retinal pigmented epithelium in C57BL/6 mouse model. Lineage negative cells were isolated from human umbilical cord blood, whereas neurospheres were derived from CE of post-aborted human eyeballs. The cells were then transplanted into subretinal space to study their effect on injury. Markers of neurotropic factors, retina, apoptosis, and proliferation were analyzed after injury and transplantation. mRNA expression was also analyzed by real-time polymerase chain reaction at 1 week, and 3-month immunohistochemistry was evaluated at 1-week time point. CE cell transplantation showed enhanced differentiation of rods and retinal glial cells. However, Lin-ve cells exerted paracrine-dependent modulation of neurotrophic factors, which is possibly mediated by antiapoptotic and proliferative effects. In conclusion, CE transplantation showed superior regenerative outcome in comparison to Lin-ve SC for rescue of artificially injured rodent retinal cells. It is imperative that this source for transplantation may be extensively studied in various doses and additional retinal degeneration models for prospective clinical applications.
Keywords: ciliary epithelium cells; laser injury; lineage negative stem cells; retinal degeneration; subretinal; umbilical cord blood.
Conflict of interest statement
Figures




Similar articles
-
Human Fetal Pigmented Ciliary Epithelium Stem Cells have Regenerative Capacity in the Murine Retinal Degeneration Model of Laser Injury.Curr Neurovasc Res. 2019;16(3):187-193. doi: 10.2174/1567202616666190618123931. Curr Neurovasc Res. 2019. PMID: 31258084
-
Alteration of Neurotrophic Factors After Transplantation of Bone Marrow Derived Lin-ve Stem Cell in NMDA-Induced Mouse Model of Retinal Degeneration.J Cell Biochem. 2017 Jul;118(7):1699-1711. doi: 10.1002/jcb.25827. Epub 2017 Jan 11. J Cell Biochem. 2017. PMID: 27935095
-
Neurotrophic Factors Mediated Activation of Astrocytes Ameliorate Memory Loss by Amyloid Clearance after Transplantation of Lineage Negative Stem Cells.Mol Neurobiol. 2019 Dec;56(12):8420-8434. doi: 10.1007/s12035-019-01680-z. Epub 2019 Jun 27. Mol Neurobiol. 2019. PMID: 31250384
-
Regeneration of the retina: toward stem cell therapy for degenerative retinal diseases.BMB Rep. 2015 Apr;48(4):193-9. doi: 10.5483/bmbrep.2015.48.4.276. BMB Rep. 2015. PMID: 25560700 Free PMC article. Review.
-
A Promising Tool in Retina Regeneration: Current Perspectives and Challenges When Using Mesenchymal Progenitor Stem Cells in Veterinary and Human Ophthalmological Applications.Stem Cell Rev Rep. 2017 Oct;13(5):598-602. doi: 10.1007/s12015-017-9750-4. Stem Cell Rev Rep. 2017. PMID: 28643176 Free PMC article. Review.
Cited by
-
Rewiring the Spine-Cutting-Edge Stem Cell Therapies for Spinal Cord Repair.Int J Mol Sci. 2025 May 23;26(11):5048. doi: 10.3390/ijms26115048. Int J Mol Sci. 2025. PMID: 40507858 Free PMC article. Review.
-
Coping Strategy, Life Style and Health Status During Phase 3 of Indian National Lockdown for COVID-19 Pandemic-A Pan-India Survey.Front Public Health. 2022 May 18;10:814328. doi: 10.3389/fpubh.2022.814328. eCollection 2022. Front Public Health. 2022. PMID: 35664115 Free PMC article.
-
Organoid-derived human retinal progenitor cells promote early dedifferentiation of Müller glia in Royal College of Surgeons rats.Int J Ophthalmol. 2023 Apr 18;16(4):483-498. doi: 10.18240/ijo.2023.04.01. eCollection 2023. Int J Ophthalmol. 2023. PMID: 37077494 Free PMC article.
-
Retinal cell transplantation in retinitis pigmentosa.Taiwan J Ophthalmol. 2021 Dec 6;11(4):336-347. doi: 10.4103/tjo.tjo_48_21. eCollection 2021 Oct-Dec. Taiwan J Ophthalmol. 2021. PMID: 35070661 Free PMC article. Review.
-
Stem Cell-Based Approaches for Spinal Cord Injury: The Promise of iPSCs.Biology (Basel). 2025 Mar 20;14(3):314. doi: 10.3390/biology14030314. Biology (Basel). 2025. PMID: 40136570 Free PMC article. Review.
References
-
- Dietrich KC. Aircrew and handheld laser exposure. Aerosp Med Hum Perform. 2017;88(11):1040–1042. - PubMed
-
- Sliney DH. Risks of occupational exposure to optical radiation. Med Lav. 2006;97(2):215–220. - PubMed
-
- Orlacchio A, Bernardi G, Orlacchio A, Martino S. Stem cells: an overview of the current status of therapies for central and peripheral nervous system diseases. Curr Med Chem. 2010;17(7):595–608. - PubMed
-
- Hill AJ, Zwart I, Tam HH, Chan J, Navarrete C, Jen LS, Navarrete R. Human umbilical cord blood-derived mesenchymal stem cells do not differentiate into neural cell types or integrate into the retina after intravitreal grafting in neonatal rats. Stem Cells Dev. 2009;18(3):399–409. - PubMed
-
- Muthaian R, Minhas G, Anand A. Pathophysiology of stroke and stroke-induced retinal ischemia: emerging role of stem cells. J Cell Physiol. 2012;227(3):1269–1279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

