Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (Prevention of migraine via intravenous ALD403 safety and efficacy-2) study
- PMID: 33023473
- PMCID: PMC7539382
- DOI: 10.1186/s10194-020-01186-3
Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (Prevention of migraine via intravenous ALD403 safety and efficacy-2) study
Abstract
Background: PROMISE-2 was a phase 3, randomized, double-blind, placebo-controlled study that evaluated the efficacy and safety of repeat intravenous (IV) doses of the calcitonin gene-related peptide-targeted monoclonal antibody eptinezumab (ALD403) for migraine prevention in adults with chronic migraine. This report describes the results of PROMISE-2 through 24 weeks of treatment.
Methods: Patients received up to two 30-min IV administrations of eptinezumab 100 mg, 300 mg, or placebo separated by 12 weeks. Patients recorded migraine and headache endpoints in a daily eDiary. Additional assessments, including patient-reported outcomes, were performed at regularly scheduled clinic visits throughout the 32-week study period (screening, day 0, and weeks 2, 4, 8, 12, 16, 20, 24, and 32).
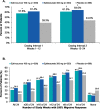
Results: A total of 1072 adults received treatment: eptinezumab 100 mg, n = 356; eptinezumab 300 mg, n = 350; placebo, n = 366. The reduction in mean monthly migraine days observed during the first dosing interval (100 mg, - 7.7 days; 300 mg, - 8.2 days; placebo, - 5.6 days) was further decreased after an additional dose (100 mg, - 8.2 days; 300 mg, - 8.8 days; placebo, - 6.2 days), with both doses of eptinezumab demonstrating consistently greater reductions from baseline compared to placebo. The ≥50% and ≥ 75% migraine responder rates (MRRs) increased after a second dose, with more eptinezumab-treated patients experiencing migraine response than placebo patients (≥50% MRRs weeks 13-24: 100 mg, 61.0%; 300 mg, 64.0%; placebo, 44.0%; and ≥ 75% MRRs weeks 13-24: 100 mg, 39.3%; 300 mg, 43.1%; placebo, 23.8%). The percentages of patients who improved on patient-reported outcomes, including the Headache Impact Test and Patient Global Impression of Change, increased following the second dose administration at week 12, and were greater with eptinezumab than with placebo at all time points. No new safety concerns were identified with the second dose regarding the incidence, nature, and severity of treatment-emergent adverse events.
Conclusion: Eptinezumab 100 mg or 300 mg administered IV at day 0 and repeated at week 12 provided sustained migraine preventive benefit over a full 24 weeks and demonstrated an acceptable safety profile in patients with chronic migraine.
Trial registration: ClinicalTrials.gov (Identifier: NCT02974153 ). Registered November 23, 2016.
Keywords: Chronic migraine; Efficacy; Eptinezumab; Safety.
Conflict of interest statement
SS: Receives or has received, as a consultant and/or advisory panel member, honoraria from Allergan, Amgen, Avanir Pharmaceuticals, Cefaly, Curelator, Dr. Reddy’s Laboratories, Egalet Corporation, electroCore Medical, eNeura, Lilly USA, Lundbeck Seattle Biopharmaceuticals, Medscape, National Institute of Neurological Disorders and Stroke, Satsuma Pharmaceuticals, Supernus Pharmaceuticals, Teva Pharmaceuticals, Theranica, and Trigemina, and reports consulting fees from Alder, Allergan, Amgen, Avanir, Curelator, Dr. Reddy’s Laboratories, electroCore, Eli Lilly, eNeura, Medscape, National Institute of Neurological Disorders and Stroke, Supernus, Teva, Theranica, and Trigemina.
MD: Advisory boards and a consultant for Amgen, Lundbeck Seattle Biopharmaceuticals, Promius Pharma, Teva Pharmaceutical Industries; advisory boards for Avanir Pharmaceuticals, Depomed, Eli Lilly, Supernus Pharmaceuticals, Upsher-Smith Laboratories; speakers’ bureaus for Amgen, Avanir Pharmaceuticals, Depomed, Pernix Therapeutics, Supernus Pharmaceuticals, Teva Pharmaceutical Industries; Board of Directors for the National Headache Foundation and the Diamond Headache Clinic Research and Educational Foundation.
NAH: Serves on speaker’s bureaus for Amgen, Eli Lilly, and electroCore, and serves on advisory boards for Amgen, Eli Lilly, Lundbeck Chicago Biopharmaceuticals, and Zosano Pharma.
DB: Full-time employee of Alder BioPharmaceuticals (currently known as Lundbeck Seattle BioPharmaceuticals) at the time of the study; Contracted service provider during manuscript development.
RC: Full-time employee of Lundbeck Seattle BioPharmaceuticals.
JH: Contracted service provider of biostatistical resources to Lundbeck Seattle BioPharmaceuticals.
BA: Full-time employee of Alder BioPharmaceuticals (currently known as Lundbeck Seattle BioPharmaceuticals) at the time of the study; Contracted service provider during manuscript development.
SP: Full-time employee of Lundbeck Seattle BioPharmaceuticals.
BS: Full-time employee of Lundbeck Seattle BioPharmaceuticals at the time of the study and during manuscript development.
JS: Full-time employee of Alder BioPharmaceuticals (currently known as Lundbeck Seattle BioPharmaceuticals) at the time of the study; Contracted service provider during manuscript development.
Figures




References
-
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

