Basal Ti level in the human placenta and meconium and evidence of a materno-foetal transfer of food-grade TiO2 nanoparticles in an ex vivo placental perfusion model
- PMID: 33023621
- PMCID: PMC7541303
- DOI: 10.1186/s12989-020-00381-z
Basal Ti level in the human placenta and meconium and evidence of a materno-foetal transfer of food-grade TiO2 nanoparticles in an ex vivo placental perfusion model
Abstract
Background: Titanium dioxide (TiO2) is broadly used in common consumer goods, including as a food additive (E171 in Europe) for colouring and opacifying properties. The E171 additive contains TiO2 nanoparticles (NPs), part of them being absorbed in the intestine and accumulated in several systemic organs. Exposure to TiO2-NPs in rodents during pregnancy resulted in alteration of placental functions and a materno-foetal transfer of NPs, both with toxic effects on the foetus. However, no human data are available for pregnant women exposed to food-grade TiO2-NPs and their potential transfer to the foetus. In this study, human placentae collected at term from normal pregnancies and meconium (the first stool of newborns) from unpaired mothers/children were analysed using inductively coupled plasma mass spectrometry (ICP-MS) and scanning transmission electron microscopy (STEM) coupled to energy-dispersive X-ray (EDX) spectroscopy for their titanium (Ti) contents and for analysis of TiO2 particle deposition, respectively. Using an ex vivo placenta perfusion model, we also assessed the transplacental passage of food-grade TiO2 particles.
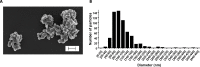
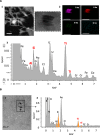
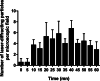
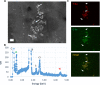
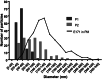
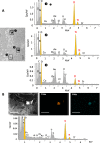
Results: By ICP-MS analysis, we evidenced the presence of Ti in all placentae (basal level ranging from 0.01 to 0.48 mg/kg of tissue) and in 50% of the meconium samples (0.02-1.50 mg/kg), suggesting a materno-foetal passage of Ti. STEM-EDX observation of the placental tissues confirmed the presence of TiO2-NPs in addition to iron (Fe), tin (Sn), aluminium (Al) and silicon (Si) as mixed or isolated particle deposits. TiO2 particles, as well as Si, Al, Fe and zinc (Zn) particles were also recovered in the meconium. In placenta perfusion experiments, confocal imaging and SEM-EDX analysis of foetal exudate confirmed a low transfer of food-grade TiO2 particles to the foetal side, which was barely quantifiable by ICP-MS. Diameter measurements showed that 70 to 100% of the TiO2 particles recovered in the foetal exudate were nanosized.
Conclusions: Altogether, these results show a materno-foetal transfer of TiO2 particles during pregnancy, with food-grade TiO2 as a potential source for foetal exposure to NPs. These data emphasize the need for risk assessment of chronic exposure to TiO2-NPs during pregnancy.
Keywords: E171 food additive; Foetus; Human placenta; Nanoparticles; Titanium dioxide.
Conflict of interest statement
Authors declare that they have no competing interests.
Figures
References
-
- Böckmann J, Lahl H, Eckert T, Unterhalt B. Blood levels of titanium before and after oral administration of titanium dioxide. Pharmazie. 2000;55:140–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

