Clinical Evaluation of BD Veritor SARS-CoV-2 Point-of-Care Test Performance Compared to PCR-Based Testing and versus the Sofia 2 SARS Antigen Point-of-Care Test
- PMID: 33023911
- PMCID: PMC7771450
- DOI: 10.1128/JCM.02338-20
Clinical Evaluation of BD Veritor SARS-CoV-2 Point-of-Care Test Performance Compared to PCR-Based Testing and versus the Sofia 2 SARS Antigen Point-of-Care Test
Abstract
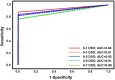
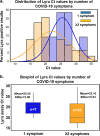
The clinical performance of the BD Veritor System for Rapid Detection of SARS-CoV-2 nucleocapsid antigen (Veritor), a chromatographic immunoassay used for SARS-CoV-2 point-of-care testing, was evaluated using nasal specimens from individuals with COVID-19 symptoms. Two studies were completed to determine clinical performance. In the first study, nasal specimens and either nasopharyngeal or oropharyngeal specimens from 251 participants with COVID-19 symptoms (≤7 days from symptom onset [DSO], ≥18 years of age) were utilized to compare Veritor with the Lyra SARS-CoV-2 PCR assay (Lyra). In the second study, nasal specimens from 361 participants with COVID-19 symptoms (≤5 DSO, ≥18 years of age) were utilized to compare performance of Veritor to that of the Sofia 2 SARS Antigen FIA test (Sofia 2). The positive, negative, and overall percent agreement (PPA, NPA, and OPA, respectively) were the primary outcomes. In study 1, the PPA for Veritor, compared to Lyra, ranged from 81.8 to 87.5% across the 0 to 1 and 0 to 6 DSO ranges. In study 2, Veritor had PPA, NPA, and OPA values of 97.4, 98.1, and 98.1%, respectively, with Sofia 2. Discordant analysis showed one Lyra positive missed by Veritor and five Lyra positives missed by Sofia 2; one Veritor positive result was negative by Lyra. Veritor met FDA emergency use authorization (EUA) acceptance criteria for SARS-CoV-2 antigen testing for the 0 to 5 and 0 to 6 DSO ranges (PPA values of 83.9% and 82.4%, respectively). Veritor and Sofia 2 showed a high degree of agreement for SARS-CoV-2 detection. The Veritor test allows for more rapid COVID-19 testing utilizing easy-to-collect nasal swabs but demonstrated <100% PPA compared to PCR.
Keywords: COVID-19; SARS-CoV-2; Sofia 2 test; Veritor test; point-of-care test.
Copyright © 2020 Young et al.
Figures

Comment in
-
Evaluation of Performance of the BD Veritor SARS-CoV-2 Chromatographic Immunoassay Test in Patients with Symptoms of COVID-19.J Clin Microbiol. 2021 Apr 20;59(5):e00260-21. doi: 10.1128/JCM.00260-21. Print 2021 Apr 20. J Clin Microbiol. 2021. PMID: 33637583 Free PMC article. No abstract available.
References
-
- Policy for Coronavirus Disease. 2019. Tests during the public health emergency (revised): immediately in effect guidance for clinical laboratories, commercial manufacturers, and food and drug administration staff, version May 11, 2020. U.S. Food and Drug Administration, Bethesda, MD: https://www.fda.gov/regulatory-information/search-fda-guidance-documents....
-
- Reusken CBEM, Broberg EK, Haagmans B, Meijer A, Corman VM, Papa A, Charrel R, Drosten C, Koopmans M, Leitmeyer K, on behalf of EVD-LabNet and ERLI-Net. 2020. Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020. Eurosurveillance 25:2000082. doi: 10.2807/1560-7917.ES.2020.25.6.2000082. - DOI - PMC - PubMed

