Regulation of Body Size and Growth Control
- PMID: 33023929
- PMCID: PMC7536854
- DOI: 10.1534/genetics.120.303095
Regulation of Body Size and Growth Control
Abstract
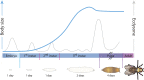
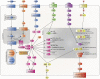
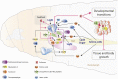
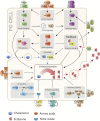
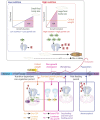
The control of body and organ growth is essential for the development of adults with proper size and proportions, which is important for survival and reproduction. In animals, adult body size is determined by the rate and duration of juvenile growth, which are influenced by the environment. In nutrient-scarce environments in which more time is needed for growth, the juvenile growth period can be extended by delaying maturation, whereas juvenile development is rapidly completed in nutrient-rich conditions. This flexibility requires the integration of environmental cues with developmental signals that govern internal checkpoints to ensure that maturation does not begin until sufficient tissue growth has occurred to reach a proper adult size. The Target of Rapamycin (TOR) pathway is the primary cell-autonomous nutrient sensor, while circulating hormones such as steroids and insulin-like growth factors are the main systemic regulators of growth and maturation in animals. We discuss recent findings in Drosophila melanogaster showing that cell-autonomous environment and growth-sensing mechanisms, involving TOR and other growth-regulatory pathways, that converge on insulin and steroid relay centers are responsible for adjusting systemic growth, and development, in response to external and internal conditions. In addition to this, proper organ growth is also monitored and coordinated with whole-body growth and the timing of maturation through modulation of steroid signaling. This coordination involves interorgan communication mediated by Drosophila insulin-like peptide 8 in response to tissue growth status. Together, these multiple nutritional and developmental cues feed into neuroendocrine hubs controlling insulin and steroid signaling, serving as checkpoints at which developmental progression toward maturation can be delayed. This review focuses on these mechanisms by which external and internal conditions can modulate developmental growth and ensure proper adult body size, and highlights the conserved architecture of this system, which has made Drosophila a prime model for understanding the coordination of growth and maturation in animals.
Keywords: DILP8; Drosophila; FlyBook; PTTH; checkpoint; critical weight; ecdysone; insulin; metamorphosis; prothoracic gland; timing.
Copyright © 2020 by the Genetics Society of America.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

