Metformin rescues Parkinson's disease phenotypes caused by hyperactive mitochondria
- PMID: 33024014
- PMCID: PMC7585014
- DOI: 10.1073/pnas.2009838117
Metformin rescues Parkinson's disease phenotypes caused by hyperactive mitochondria
Abstract
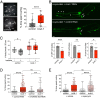
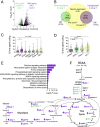
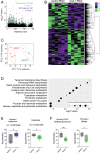
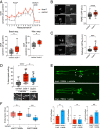
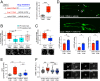
Metabolic dysfunction occurs in many age-related neurodegenerative diseases, yet its role in disease etiology remains poorly understood. We recently discovered a potential causal link between the branched-chain amino acid transferase BCAT-1 and the neurodegenerative movement disorder Parkinson's disease (PD). RNAi-mediated knockdown of Caenorhabditis elegans bcat-1 is known to recapitulate PD-like features, including progressive motor deficits and neurodegeneration with age, yet the underlying mechanisms have remained unknown. Using transcriptomic, metabolomic, and imaging approaches, we show here that bcat-1 knockdown increases mitochondrial respiration and induces oxidative damage in neurons through mammalian target of rapamycin-independent mechanisms. Increased mitochondrial respiration, or "mitochondrial hyperactivity," is required for bcat-1(RNAi) neurotoxicity. Moreover, we show that post-disease-onset administration of the type 2 diabetes medication metformin reduces mitochondrial respiration to control levels and significantly improves both motor function and neuronal viability. Taken together, our findings suggest that mitochondrial hyperactivity may be an early event in the pathogenesis of PD, and that strategies aimed at reducing mitochondrial respiration may constitute a surprising new avenue for PD treatment.
Keywords: Caenorhabditis elegans; Parkinson’s disease; branched-chain amino acid metabolism; metformin; mitochondria.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: The method used for automated quantification of curling motor behavior was filed under Patent #62/989,317: C.T.M., S.S., D.E.M., R.K., W.K., “Novel High-Throughput Screening Method for Parkinson’s Phenotypes Using C. elegans.” This patent is specifically for the screening method, not for metformin treatment of Parkinson’s disease phenotypes.
Figures
References
-
- Van Den Eeden S. K. et al. ., Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 157, 1015–1022 (2003). - PubMed
-
- de Lau L. M., Breteler M. M., Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535 (2006). - PubMed
-
- Forno L. S., Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 55, 259–272 (1996). - PubMed
-
- Spillantini M. G. et al. ., Alpha-synuclein in Lewy bodies. Nature 388, 839–840 (1997). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

