Broad-Spectrum Antimicrobial and Antibiofilm Activity of a Natural Clay Mineral from British Columbia, Canada
- PMID: 33024043
- PMCID: PMC7542368
- DOI: 10.1128/mBio.02350-20
Broad-Spectrum Antimicrobial and Antibiofilm Activity of a Natural Clay Mineral from British Columbia, Canada
Abstract
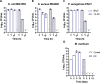
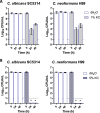
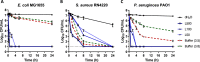
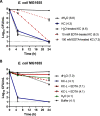
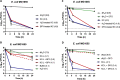
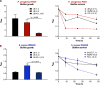
Worldwide increases in antibiotic resistance and the dearth of new antibiotics have created a global crisis in the treatment of infectious diseases. These concerns highlight the pressing need for novel antimicrobial agents. Natural clay minerals have a long history of therapeutic and biomedical applications and have lately received specific attention for their potent antimicrobial properties. In particular, Kisameet clay (KC) has strong antibacterial activity against a variety of multidrug-resistant (MDR) bacterial pathogens in vitro Here, we have extended the known spectrum of activity of KC by demonstrating its efficacy against two major fungal pathogens, Candida albicans and Cryptococcus neoformans In addition, KC also exhibits potent activity against the opportunistic bacterial pathogen Mycobacterium marinum, a model organism for M. ulcerans infection. Moreover, aqueous KC leachates (KC-L) exhibited broad-spectrum antibacterial activity, eradicated Gram-negative and Gram-positive biofilms, and prevented their formation. The mechanism(s) underlying KC antibacterial activity appears to be complex. Adjusting KC-L to neutral pH rendered it inactive, indicating a contribution of pH, although low pH alone was insufficient for its antibacterial activity. Treatment of KC minerals with cation-chelating agents such as EDTA, 2,2'-bipyridyl, and deferoxamine reduced the antibacterial activity, while supplementation of KC-L with these chelating agents eliminated the inhibitory activity. Together, the data suggest a positive role for divalent and trivalent cations, including iron and aluminum, in bacterial inhibition by KC. Collectively, these studies demonstrate the range of KC bioactivity and provide a better understanding of the mechanism underlying its antibacterial effects.IMPORTANCE The escalating emergence of multidrug-resistant (MDR) bacteria, together with the paucity of novel antimicrobial agents in antibiotic development, is recognized as a worldwide public health crisis. Kisameet clay (KC), found in British Columbia (BC), Canada, is a clay mineral with a long history of therapeutic applications among people of the First Nations. We previously reported the antibacterial activity of KC against a group of MDR clinical pathogens. Here, we demonstrate its activity against two major human-pathogenic fungal species, as well as against bacterial biofilms, which underlie many recalcitrant bacterial infections. In these studies, we also identified several geochemical characteristics of KC, such as metal ions and low pH, which are involved in its antibacterial activity. These findings provide a better understanding of the components of KC antibacterial activity and a basis for developing defined preparations of this clay mineral for therapeutic applications.
Keywords: antibacterial agent; antimicrobial clay; bacterial biofilm; clay mineral; fungal pathogen.
Copyright © 2020 Behroozian et al.
Figures

Similar articles
-
Kisameet Glacial Clay: an Unexpected Source of Bacterial Diversity.mBio. 2017 May 23;8(3):e00590-17. doi: 10.1128/mBio.00590-17. mBio. 2017. PMID: 28536287 Free PMC article.
-
Kisameet Clay Exhibits Potent Antibacterial Activity against the ESKAPE Pathogens.mBio. 2016 Jan 26;7(1):e01842-15. doi: 10.1128/mBio.01842-15. mBio. 2016. PMID: 26814180 Free PMC article.
-
Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms.Phytomedicine. 2017 Dec 15;37:14-26. doi: 10.1016/j.phymed.2017.10.021. Epub 2017 Nov 23. Phytomedicine. 2017. PMID: 29174600 Review.
-
Kisameet Clay Isolated from the Central Coast of British Columbia, Canada, Demonstrates Broad-Spectrum Antimicrobial Activity.mBio. 2016 Mar 8;7(2):e00169. doi: 10.1128/mBio.00169-16. mBio. 2016. PMID: 26956585 Free PMC article.
-
Antimicrobial Peptides as a Strategy to Combat Fungal Biofilms.Curr Top Med Chem. 2017;17(5):604-612. doi: 10.2174/1568026616666160713142228. Curr Top Med Chem. 2017. PMID: 27411323 Review.
Cited by
-
Antibacterial Activity of a Natural Clay Mineral against Burkholderia cepacia Complex and Other Bacterial Pathogens Isolated from People with Cystic Fibrosis.Microorganisms. 2023 Jan 6;11(1):150. doi: 10.3390/microorganisms11010150. Microorganisms. 2023. PMID: 36677442 Free PMC article.
-
Design SMAP29-LysPA26 as a Highly Efficient Artilysin against Pseudomonas aeruginosa with Bactericidal and Antibiofilm Activity.Microbiol Spectr. 2021 Dec 22;9(3):e0054621. doi: 10.1128/Spectrum.00546-21. Epub 2021 Dec 8. Microbiol Spectr. 2021. PMID: 34878337 Free PMC article.
-
Waste treatment innovation for infusion bottles using soil solution.PLoS One. 2022 Aug 22;17(8):e0273394. doi: 10.1371/journal.pone.0273394. eCollection 2022. PLoS One. 2022. PMID: 35994450 Free PMC article.
-
Impact of dietary Biocide clay on growth, physiological status, and histological indicators of the liver and digestive tract in Nile tilapia (Oreochromis niloticus).Sci Rep. 2025 Feb 13;15(1):5311. doi: 10.1038/s41598-025-89042-9. Sci Rep. 2025. PMID: 39939694 Free PMC article.
-
Geophagia among pregnant women: evaluating the microbiological and toxicological safety of calabash chalk and its implications on maternal health.Environ Geochem Health. 2025 Jul 30;47(9):347. doi: 10.1007/s10653-025-02656-w. Environ Geochem Health. 2025. PMID: 40736606 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
