CPX-351 treatment in secondary acute myeloblastic leukemia is effective and improves the feasibility of allogeneic stem cell transplantation: results of the Italian compassionate use program
- PMID: 33024084
- PMCID: PMC7538937
- DOI: 10.1038/s41408-020-00361-8
CPX-351 treatment in secondary acute myeloblastic leukemia is effective and improves the feasibility of allogeneic stem cell transplantation: results of the Italian compassionate use program
Abstract
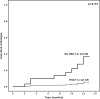
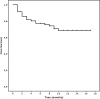
Secondary acute myeloid leukemia (sAML) poorly responds to conventional treatments and allogeneic stem cell transplantation (HSCT). We evaluated toxicity and efficacy of CPX-351 in 71 elderly patients (median age 66 years) with sAML enrolled in the Italian Named (Compassionate) Use Program. Sixty days treatment-related mortality was 7% (5/71). The response rate at the end of treatment was: CR/CRi in 50/71 patients (70.4%), PR in 6/71 (8.5%), and NR in 10/71 (19.7%). After a median follow-up of 11 months relapse was observed in 10/50 patients (20%) and 12 months cumulative incidence of relapse (CIR) was 23.6%. Median duration of response was not reached. In competing risk analysis, CIR was reduced when HSCT was performed in first CR (12 months CIR of 5% and 37.4%, respectively, for patients receiving (=20) or not (=30) HSCT, p = 0.012). Twelve-months OS was 68.6% (median not reached). In landmark analysis, HSCT in CR1 was the only significant predictor of longer survival (12 months OS of 100 and 70.5%, for patients undergoing or not HSCT in CR1, respectively, p = 0.011). In conclusion, we extend to a real-life setting, the notion that CPX is an effective regimen for high risk AML patients and may improve the results of HSCT.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Miesner M, et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: a comparison of 408 cases classified as “AML not otherwise specified”(AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC) Blood. 2010;116:2742–2751. doi: 10.1182/blood-2010-04-279794. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

