Hypothesis of design of biological cell robot as human immunodeficiency virus vaccine
- PMID: 33024716
- PMCID: PMC7520875
- DOI: 10.5501/wjv.v9.i3.19
Hypothesis of design of biological cell robot as human immunodeficiency virus vaccine
Abstract
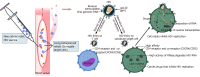
High genetic variability of human immunodeficiency virus (HIV) has been a major intractable challenge to the practical design of vaccines. But a recent pioneer study published in PNAS Xenobots, is likely to revolutionize HIV prevention as it presented the world's first living robot made of cells. In the advent of this discovery, we herein discuss the possibility of using living biological cell robots to target HIV-infected T lymphocytes, and the prospects of this approach being a new HIV vaccine. We capture the current research status and trend of advances in biological cell robots' design as a new HIV vaccine. The key differences between this novel vaccine and other HIV vaccines are highlighted.
Keywords: Biologically inspired microrobots; CD4; Human immunodeficiency virus; Human immunodeficiency virus target cell surrogate; New vaccine.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All authors have no any conflict of interests to disclose.
Figures


Similar articles
-
Vaccination of Macaques with DNA Followed by Adenoviral Vectors Encoding Simian Immunodeficiency Virus (SIV) Gag Alone Delays Infection by Repeated Mucosal Challenge with SIV.J Virol. 2019 Oct 15;93(21):e00606-19. doi: 10.1128/JVI.00606-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31413132 Free PMC article.
-
Human Immunodeficiency Virus C.1086 Envelope gp140 Protein Boosts following DNA/Modified Vaccinia Virus Ankara Vaccination Fail To Enhance Heterologous Anti-V1V2 Antibody Response and Protection against Clade C Simian-Human Immunodeficiency Virus Challenge.J Virol. 2019 Sep 30;93(20):e00934-19. doi: 10.1128/JVI.00934-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31341049 Free PMC article.
-
Comparative clonal analysis of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ and CD8+ cytolytic T lymphocytes isolated from seronegative humans immunized with candidate HIV-1 vaccines.J Exp Med. 1992 Dec 1;176(6):1531-42. doi: 10.1084/jem.176.6.1531. J Exp Med. 1992. PMID: 1460417 Free PMC article. Clinical Trial.
-
New prospects for the development of a vaccine against human immunodeficiency virus type 1. An overview.C R Acad Sci III. 1999 Nov;322(11):959-66. doi: 10.1016/s0764-4469(00)87193-0. C R Acad Sci III. 1999. PMID: 10646090 Review.
-
Multiple Approaches for Increasing the Immunogenicity of an Epitope-Based Anti-HIV Vaccine.AIDS Res Hum Retroviruses. 2015 Nov;31(11):1077-88. doi: 10.1089/AID.2015.0101. Epub 2015 Aug 13. AIDS Res Hum Retroviruses. 2015. PMID: 26149745 Review.
References
-
- Chen M, Jiang SB, Liu SW. Host cell proteins associated with HIV infection. Xibao Yu Fenzi Mianyixue Zazhi. 2009;25:662–664.
-
- Redd AD, Laeyendecker O, Kong X, Kiwanuka N, Lutalo T, Huang W, Gray RH, Wawer MJ, Serwadda D, Eshleman SH, Quinn TC Rakai Health Sciences Program. Efficiency of CCR5 coreceptor utilization by the HIV quasispecies increases over time, but is not associated with disease progression. AIDS Res Hum Retroviruses. 2012;28:289–294. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

