Mesenchymal Stem Cell-Mediated Therapy of Peripheral Artery Disease Is Stimulated by a Lamin A-Progerin Binding Inhibitor
- PMID: 33024737
- PMCID: PMC7521968
- DOI: 10.12997/jla.2020.9.3.460
Mesenchymal Stem Cell-Mediated Therapy of Peripheral Artery Disease Is Stimulated by a Lamin A-Progerin Binding Inhibitor
Abstract
Objective: Human adipose tissue-derived mesenchymal stem cells (ASCs) have been reported to promote angiogenesis and tissue repair. However, poor survival and engraftment efficiency of transplanted ASCs are the major bottlenecks for therapeutic application. The present study aims to improve the therapeutic efficacy of ASCs for peripheral artery diseases.
Methods: Hydrogen peroxide (H2O2) was used to induce apoptotic cell death in ASCs. To measure apoptosis, we used flow cytometry-based apoptosis analysis and terminal deoxynucleotidyl transferase dUTP nick end labeling staining. A murine hindlimb ischemia model was established to measure the ASC-mediated therapeutic angiogenesis and in vivo survival ability of ASCs.
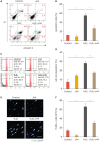
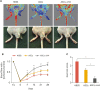
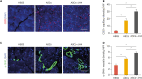
Results: We identified that the inhibitor of lamin A-progerin binding, JH4, protects ASCs against H2O2-induced oxidative stress and apoptosis. Co-administration of ASCs with JH4 improved ASC-mediated blood reperfusion recovery and limb salvage compared to that of the control group in a mouse hind limb ischemia model. Immunofluorescence showed that JH4 treatment potentiated ASC-mediated vascular regeneration via reducing ASC apoptosis post transplantation.
Conclusion: JH4 exerts anti-apoptotic effects in ASCs in conditions of oxidative stress, and contributes to the repair of ischemic hind limb injury by improving cell survival.
Keywords: Apoptosis; Mesenchymal stem cells; Oxidative stress; Peripheral artery disease.
Copyright © 2020 The Korean Society of Lipid and Atherosclerosis.
Conflict of interest statement
Conflict of Interest: The authors have no conflict of interest to declare.
Figures


Similar articles
-
Adipose-derived stem cell spheroid treated with low-level light irradiation accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia.Cytotherapy. 2017 Sep;19(9):1070-1078. doi: 10.1016/j.jcyt.2017.06.005. Epub 2017 Jul 21. Cytotherapy. 2017. PMID: 28739168
-
Curcumin-induced heme oxygenase-1 expression prevents H2O2-induced cell death in wild type and heme oxygenase-2 knockout adipose-derived mesenchymal stem cells.Int J Mol Sci. 2014 Oct 8;15(10):17974-99. doi: 10.3390/ijms151017974. Int J Mol Sci. 2014. PMID: 25299695 Free PMC article.
-
Vascular regeneration effect of adipose-derived stem cells with light-emitting diode phototherapy in ischemic tissue.Lasers Med Sci. 2015 Feb;30(2):533-41. doi: 10.1007/s10103-014-1699-9. Epub 2015 Jan 8. Lasers Med Sci. 2015. PMID: 25567209
-
Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells.Int J Mol Sci. 2021 Oct 8;22(19):10890. doi: 10.3390/ijms221910890. Int J Mol Sci. 2021. PMID: 34639228 Free PMC article. Review.
-
Translational research aiming to improve survival of ovarian tissue transplants using adipose tissue-derived stem cells.Acta Obstet Gynecol Scand. 2019 May;98(5):665-671. doi: 10.1111/aogs.13610. Epub 2019 Apr 13. Acta Obstet Gynecol Scand. 2019. PMID: 30891730 Review.
Cited by
-
Modulation of Mesenchymal Stem Cells for Enhanced Therapeutic Utility in Ischemic Vascular Diseases.Int J Mol Sci. 2021 Dec 27;23(1):249. doi: 10.3390/ijms23010249. Int J Mol Sci. 2021. PMID: 35008675 Free PMC article. Review.
References
-
- Song P, Rudan D, Zhu Y, Fowkes FJ, Rahimi K, Fowkes FG, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. - PubMed
-
- Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Lüders F, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36:932–938. - PubMed
-
- Patel SD, Biasi L, Paraskevopoulos I, Silickas J, Lea T, Diamantopoulos A, et al. Comparison of angioplasty and bypass surgery for critical limb ischaemia in patients with infrapopliteal peripheral artery disease. Br J Surg. 2016;103:1815–1822. - PubMed
-
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

