This is a preprint.
SARS-CoV-2 D614G Variant Exhibits Enhanced Replication ex vivo and Earlier Transmission in vivo
- PMID: 33024969
- PMCID: PMC7536872
- DOI: 10.1101/2020.09.28.317685
SARS-CoV-2 D614G Variant Exhibits Enhanced Replication ex vivo and Earlier Transmission in vivo
Update in
-
SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo.Science. 2020 Dec 18;370(6523):1464-1468. doi: 10.1126/science.abe8499. Epub 2020 Nov 12. Science. 2020. PMID: 33184236 Free PMC article.
Abstract
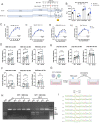
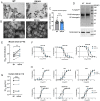
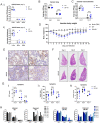
The D614G substitution in the S protein is most prevalent SARS-CoV-2 strain circulating globally, but its effects in viral pathogenesis and transmission remain unclear. We engineered SARS-CoV-2 variants harboring the D614G substitution with or without nanoluciferase. The D614G variant replicates more efficiency in primary human proximal airway epithelial cells and is more fit than wildtype (WT) virus in competition studies. With similar morphology to the WT virion, the D614G virus is also more sensitive to SARS-CoV-2 neutralizing antibodies. Infection of human ACE2 transgenic mice and Syrian hamsters with the WT or D614G viruses produced similar titers in respiratory tissue and pulmonary disease. However, the D614G variant exhibited significantly faster droplet transmission between hamsters than the WT virus, early after infection. Our study demonstrated the SARS-CoV2 D614G substitution enhances infectivity, replication fitness, and early transmission.
Figures
References
-
- Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A. S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.-R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M. M., Aepfelbacher M., Püschel K., Kluge S., Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Annals of Internal Medicine. 173, 268–277 (2020). - PMC - PubMed
-
- Hou Y. J., Okuda K., Edwards C. E., Martinez D. R., Asakura T., Dinnon K. H., Kato T., Lee R. E., Yount B. L., Mascenik T. M., Chen G., Olivier K. N., Ghio A., Tse L. V., Leist S. R., Gralinski L. E., Schäfer A., Dang H., Gilmore R., Nakano S., Sun L., Fulcher M. L., Livraghi-Butrico A., Nicely N. I., Cameron M., Cameron C., Kelvin D. J., de Silva A., Margolis D. M., Markmann A., Bartelt L., Zumwalt R., Martinez F. J., Salvatore S. P., Borczuk A., Tata P. R., Sontake V., Kimple A., Jaspers I., O’Neal W. K., Randell S. H., Boucher R. C., Baric R. S., SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 182, 429–446.e14 (2020). - PMC - PubMed
-
- Korber B., Fischer W. M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E. E., Bhattacharya T., Foley B., Hastie K. M., Parker M. D., Partridge D. G., Evans C. M., Freeman T. M., de Silva T. I., Angyal A., Brown R. L., Carrilero L., Green L. R., Groves D. C., Johnson K. J., Keeley A. J., Lindsey B. B., Parsons P. J., Raza M., Rowland-Jones S., Smith N., Tucker R. M., Wang D., Wyles M. D., McDanal C., Perez L. G., Tang H., Moon-Walker A., Whelan S. P., LaBranche C. C., Saphire E. O., Montefiori D. C., Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 182, 812–827.e19 (2020). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
